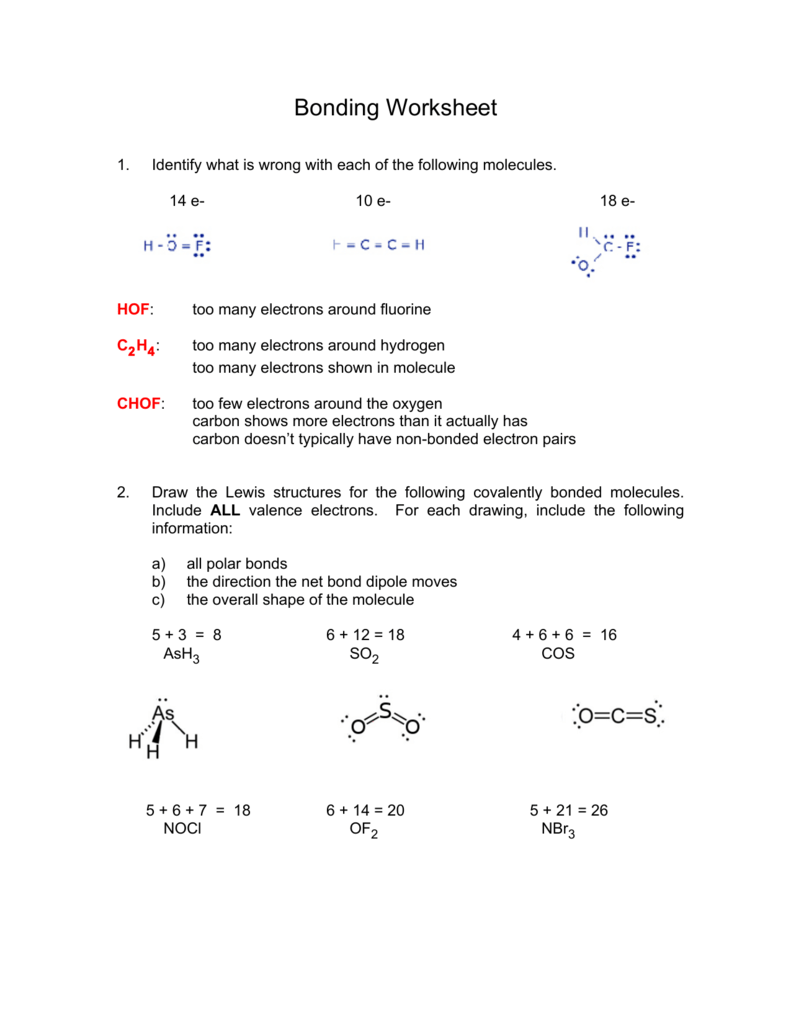
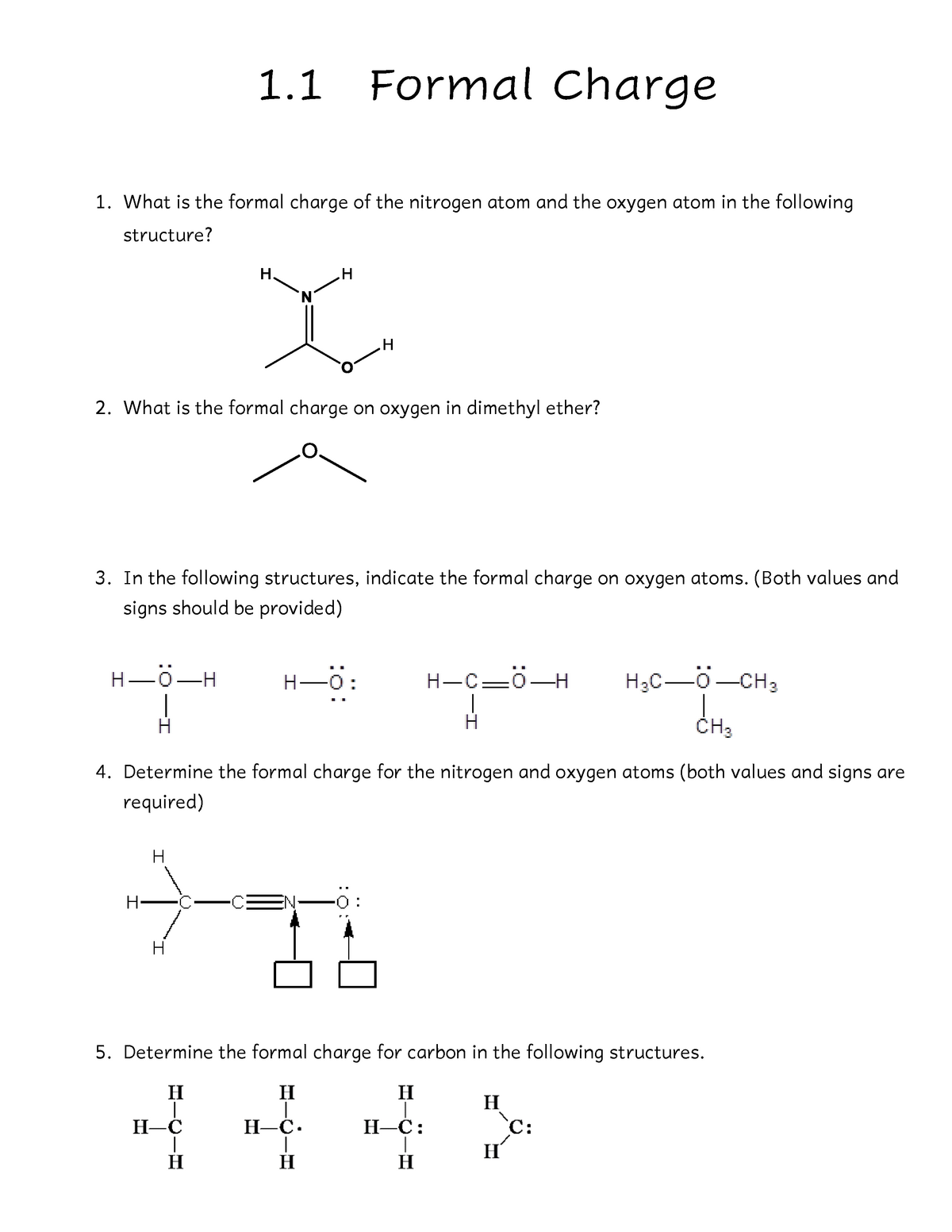
39 formal charge practice worksheet
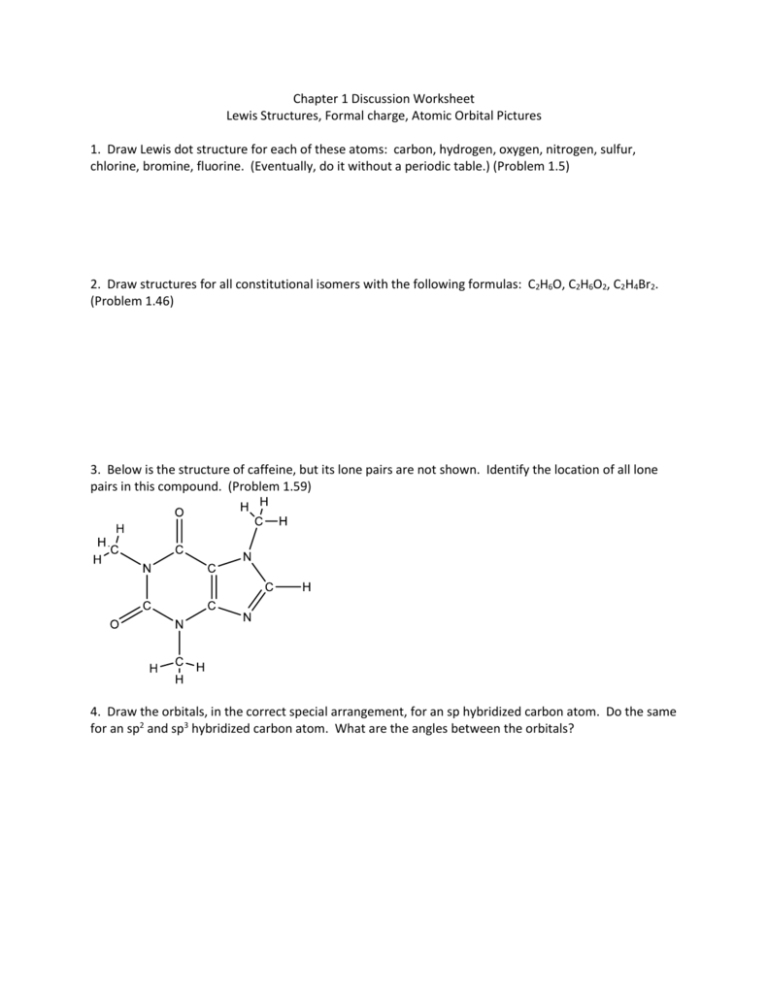
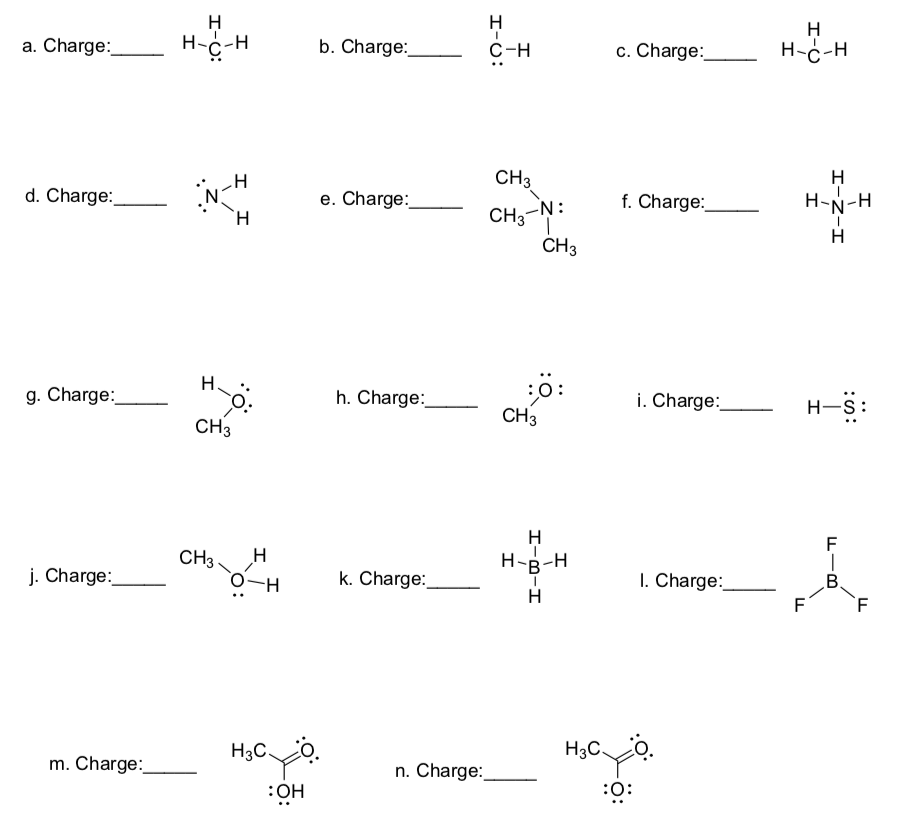
3. Draw structures for the following molecules that include any formal charges and lone pairs where needed. Each of the molecules has either single bonds or double bonds between atoms and there may be ionic bonds involved in some of the structures. 4. Indicate the hybridization of each of the C, N, and O atoms in the following molecules. 5.
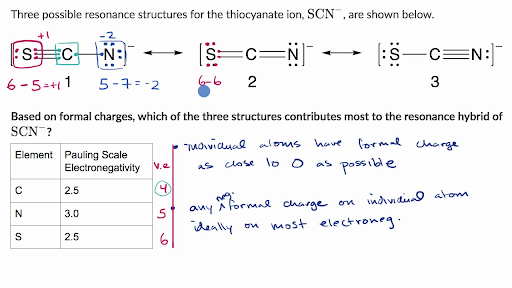
Formal Charge When more than one resonance structure is possible, the determination of the best structure can made based on the formal charges in the structure. The calculation of the formal charge is given by: # valence electrons - 1/2 shared electrons - lone pair electrons We determined that the structure of BF3 is an exception to the octet rule
A video of formal charge practice problems (from easy to difficult) with clear, concise answers and explanations.Calculating the formal charges for a molecul...
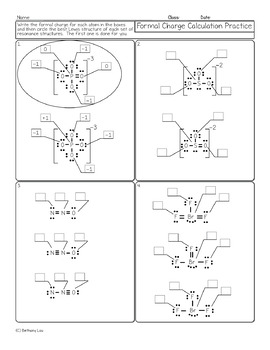
Formal charge practice worksheet
Formal Charges. Questions 1 through 8 refer to the group of structures shown on the left. Enter appropriate letters from A through K in each answer box. If no structure fits the property enter the letter X. Do not enter superfluous characters, since they will be counted as incorrect answers..
The formal charge is the charge a bonded atom in a molecule would have if the electrons in its bonding pairs were shared evenly. Identifying the formal charge is useful because it can help predict ...
Formal charge is the actual charge on an individual atom within a larger molecule or polyatomic ion. The sum of formal charges on any molecule or ion results in the net overall charge. This concept is simple enough for small ions. Chloride obviously has a negative charge. Even the negative charge on the hydroxide oxygen is simple to understand.
Formal charge practice worksheet.
Subtract this number from the number of valence electrons for the neutral atom. This gives the formal charge:Br: 7 - 7 = 0Cl: 7 - 7 = 0. All atoms in BrCl 3 have a formal charge of zero, and the sum of the formal charges totals zero, as it must in a neutral molecule. Determine the formal charge for each atom in NCl 3.
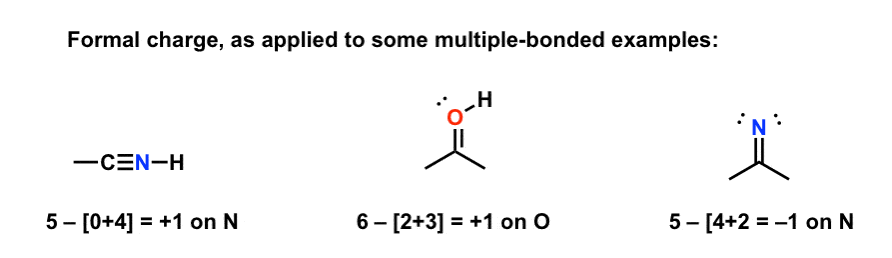
FORMAL CHARGES QUIZ Assign the correct formal charge to the specified atom in the molecules below. 1.What is the formal charge on the oxygen (note: double bonds count as four shared electrons) +1 -1 zero. 2.What is the formal charge on the carbon atom +1 -1 zero. 3. What is the formal charge on the oxygen ...
Displaying top 8 worksheets found for - Formal Charges. Some of the worksheets for this concept are Formal charges draw the lewis structure for the, Chm1045formal charges and resonance structures name, Work 14, University of illinois urbana champaign, Chem1101 work 6 lewis structures model 1 simple, Lewis structure work, Formal charge and resonance block, Covalent bonds and lewis structures.
Covalent Bonding in H2 H. .H Two hydrogen atoms, each with 1 electron, can share those electrons in a covalent bond. H:H •Sharing the electron pair gives each hydrogen an
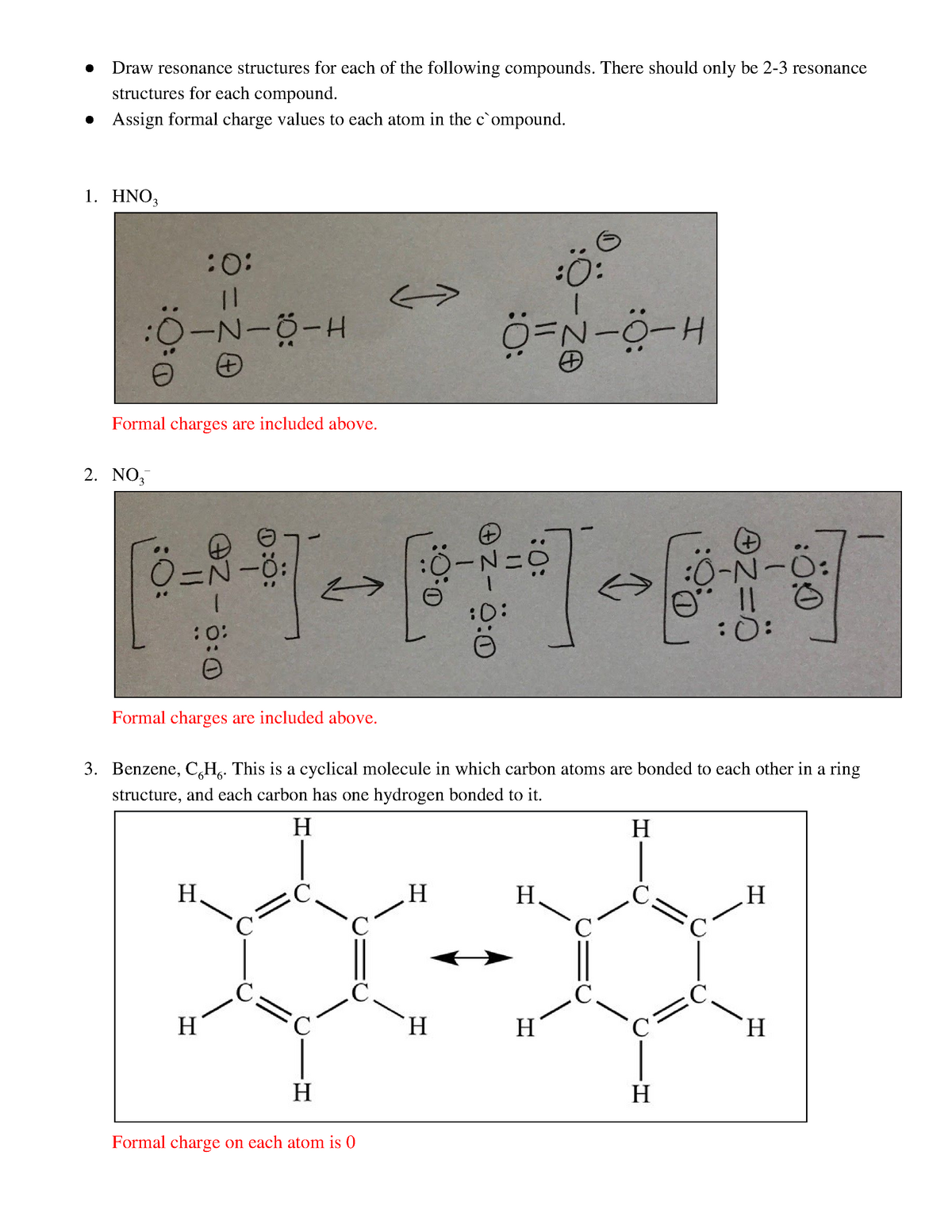
Formal Charge Worksheet / Chem 314 Beauchamp y:\files\classes\0 Organic Topics - latest\315 topics\20 315 lecture notes, 7-9-15\314 Review Problems\04 formal charge, 2D, resonance, probs & answers, 6p.doc Add in any resonance details and write formal charge next to the atom with the charge, if present. CH3NO2 a. CH2N2 b. NH3CH2CO2 c. d. CH3N3 e ...
Chem 350 Jasperse Ch. 1 Formal Charge 3 Formal Charge Practice (Section 1-7) 1. Assign any formal charges to atoms that need them: CCC H H H H H H H CCC H H H H H H H H C C C H HHH HCN H HH H CN H HH H H HCO H H HCO H H H HCO H HH H 2. Fill in lone pairs on any atoms that need them (whether atoms with formal charge or
Formal Charge Practice Worksheet For High School. Formal Charge Multiple Choice Question Learn How To. 45 Lewis And Formal Charge Chemistry Libretexts. Valency And Formal Charges In Organic Chemistry. Solved Determine The Formal Charge On Each Atom In The St.
Email. Resonance and formal charge. Resonance. Formal charge. Worked example: Using formal charges to evaluate nonequivalent resonance structures. Practice: Resonance and formal charge. This is the currently selected item. Next lesson. VSEPR.
LEWIS STRUCTURES PRACTICE WORKSHEET Draw the Lewis Structures for each of the following molecules. If you are not sure if your structure is correct, do a formal charge check. You should consult the Lewis structure rules and a periodic table while doing this exercise. A periodic table will be available for the exam, but the list of rules will ...
Valence • Two of the most important factors that provide a first order evaluation of the nature of a covalent molecule arethe electron count (cf.theoctet & eighteen‐electron rules) andthe valence of each atom. • Whereas the term electron count is self‐evident (i.e., the total number electrons in the valence shell of an atom in a molecule) andused consistently, the word valence (aka ...
Formal charge is an accounting procedure. It allows chemists to determine the location of charge in a molecule as well as compare how good a Lewis structure might be. The formula for calculating formal charge is shown below: Formal Charge = # valence e- - ( # non-bonding e- + ½ # bonding e- ) Note: the formal charges must add up to the net ...
Formal Charge Questions. In order to be most effective for you, try to answer these questions before you look at the answers! You might need a periodic table to help you here.
Lewis structure and formal charge worksheet with answers Give the name of the electronic arrangement and the name for the molecular geometry for each of the species in question #3. Check Your Answers Looking at the structure of a molecule can help us to understand or to predict the behaviour of that compound. ... Lewis structures worksheet ...
This worksheet and quiz will let you practice the following skills: Reading comprehension - ensure that you draw the most important information from the related how to calculate formal charge ...
Practice Problems. Answer the following questions and check your answers below. These problems are for practice only will not be graded. Be sure you know how to draw correct Lewis Dot Structures and are able to correctly predict the electronic arrangement and molecular geometry before going on to the lab assignment.
Formal charge = Nv.e. - Nus.e. - ½ Nb.e. The formal charge is a hypothetical charge assigned from the dot structure. The formal charges in a structure tell us the "quality" of the dot structure. Some practice of assigning formal charge is necessary before you master this technique.
7. Watch for Formal Charges and Changes in Formal Charge • If an atom's charge gets more positive ⇒ it's donating/losing an electron pair ⇒ arrow must emanate from that atom or one of it's associated bonds. There are two "more positive" transactions: • When an anion becomes neutral. In this case, an arrow will emanate
Practice putting formal charges on polyatomic ions.
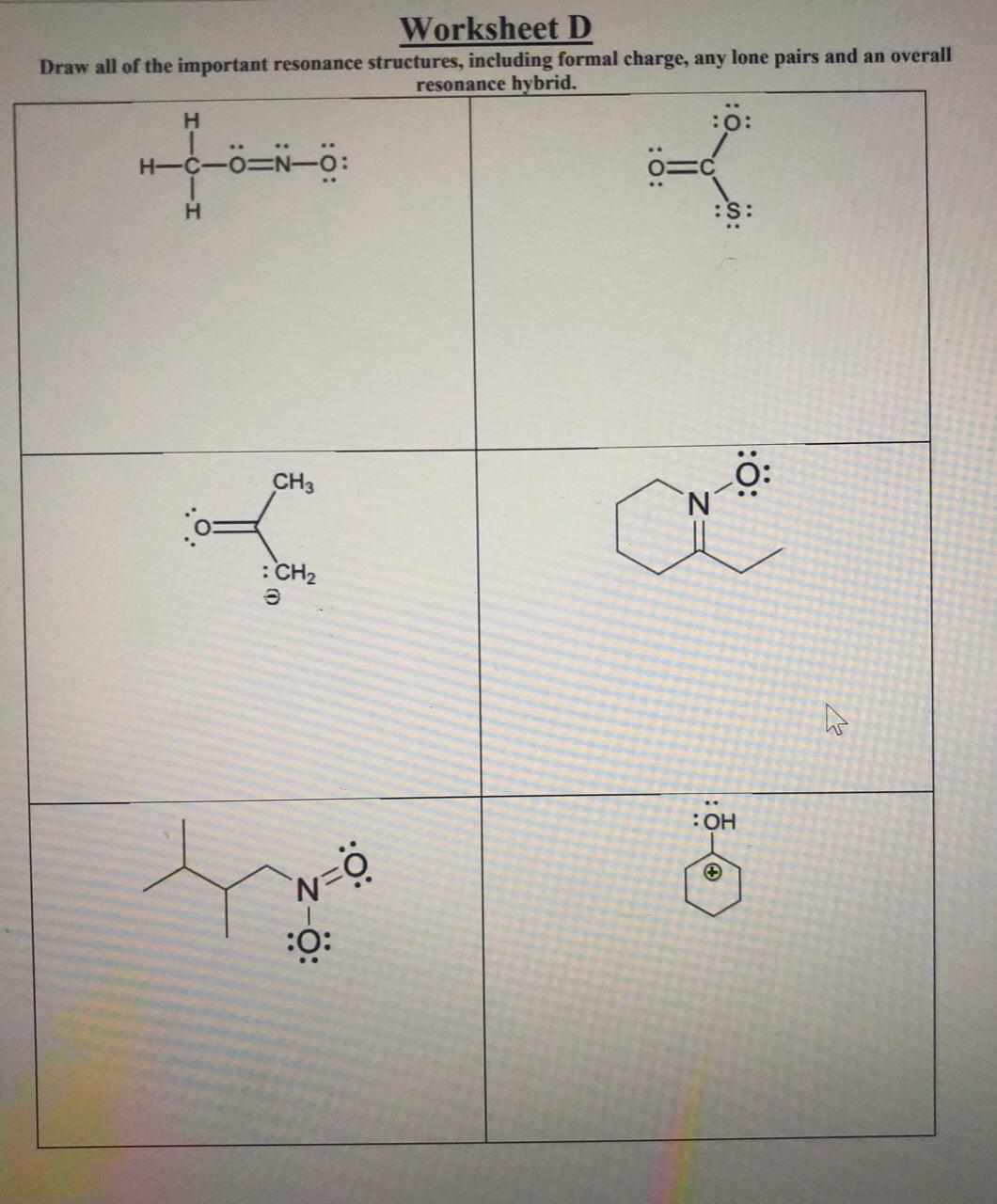
Discussion Worksheet #1 Partial Answers Resonance Skill 1: Drawing significant resonance structures Identify and use bonding patterns in molecules to draw acceptable resonance structures Check structures to ensure proper formal charge Identify which resonance structures are major, which are significant, and which are insignificant ...
• The Formal Charge is a mathematical summation of the number of actual electrons associated with an atom in a molecule. "Electronic Bookkeeping" • The Formal Charge is all determined relative to the number of valence electrons an atom would have in the ground state. ...
Formal charge on Cl atom of HClO 4 ion: 7 - 8/2 - 0 = 3. Formal charge on S atom of HSO 4 - ion: 6 - 8/2 - 0 = 2. Fun Facts On Formal Charge . In organic chemistry, convention governs that formal charge is essential for depicting a complete and correct Lewis-Kekul é structure. However, the same does not apply to inorganic chemistry.
In this activity we will learn how to draw Lewis structures, calculate formal charge and examine resonance. We will also observe the Octet rule and exceptions to this rule. AP Chem. Lewis Dot Structure Worksheet. Write Lewis Dot Structures and calculate the formal charges for the following compounds: 1. SO2 18. H2O2. CO3-2 19. C2H6. NH3 20. CH3OH
Lewis dot structures, Resonance, Bond order, Bond enthalpies, Formal charge: Organic (Part I) Help Sheet #12 Organic (Part I) Functional Groups (Part I), Drawing molecules, Addition reactions (if covered), Naming molecules, Geometric isomers (if covered), Petroleum Organic content can vary: End of Unit #1








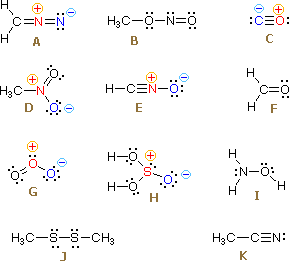














0 Response to "39 formal charge practice worksheet"
Post a Comment