41 valence electron worksheet lewis structures
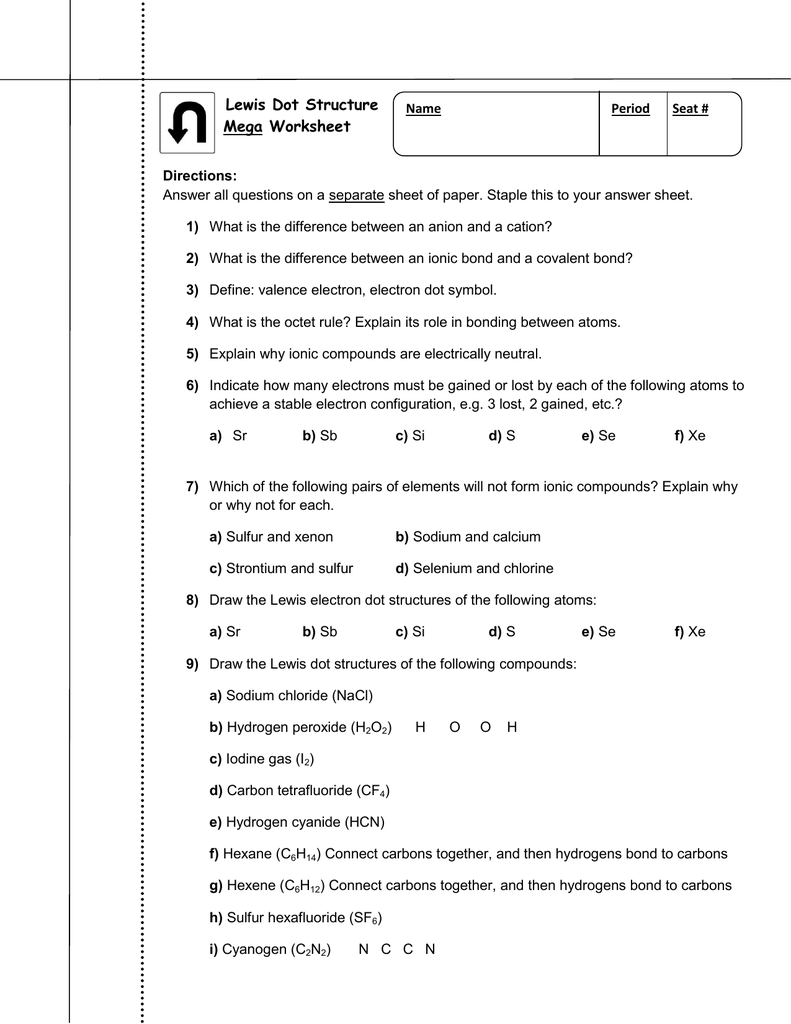
Combine carbon (4 valence electrons) and four fluorines (7 valence electrons each) to write a Lewis structure for CF4. :F:: .. .. C :F:: .. .. :F: .... :FF:: .. .. .. The octet rule is satisfied for carbon and each fluorine. Example It is common practice to represent a covalent bond by a line. We can rewrite :F:: .. .. C :F:: .. .. :F: ...
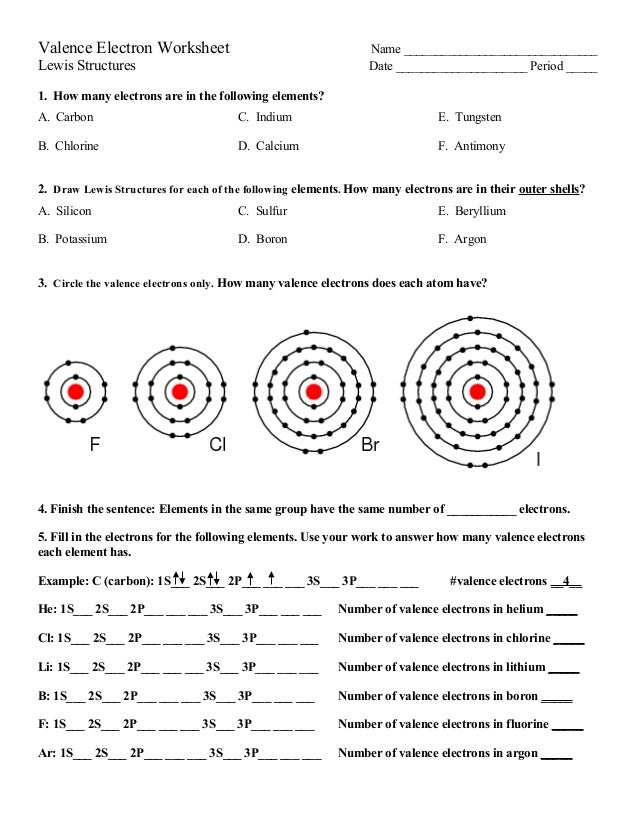
Valence Electron Worksheet Name _____ Lewis Structures Date _____ Period _____ 1. How many TOTAL electrons are in the following elements? Write the electron configuration for each element. A. Carbon. B. Chlorine. C. Indium. D. Calcium. E. Tungsten. F. Antimony 2. Draw Lewis Structures for each of the following elements. ...
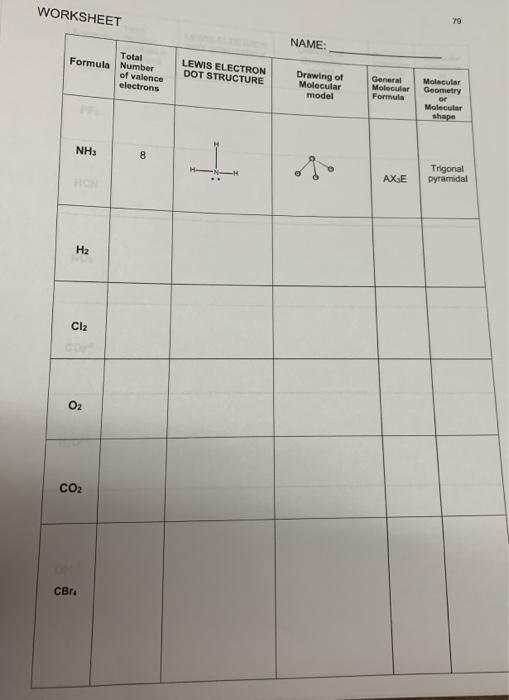
Valence electrons and lewis dot structure worksheet answers. For each molecule on the worksheet the lewis dot structure the number of valence electrons the electronarrangement e a and the molecular geometry m g are given respectively.
Valence electron worksheet lewis structures
Valence Electron Worksheet Name _____ Lewis Structures Date _____ Period _____ 1. How many electrons are in the following elements? A. Carbon. B. Chlorine. C. Indium. D. Calcium. E. Tungsten. F. Antimony. 2. Draw Lewis Struc. tures for each of the following ...
Valence Electron Worksheet Name _____ Lewis Structures Date _____ Period _____ 1. Potassium has one valence electron. C-O Valence MO electron configuration is 2s 2 2s 2 2p 2 2p 4 Bond order 8-22 3 Download Save 141 Valence Bond Molecular Orbital Theory Worksheet key. Admin October 15 2020. Special Right Triangles 45 45 90 Worksheets.
valence electrons Lewis Structure Steric Number Electron Group Geometry Molecular Geometry Hybridization Ex: H2O 8 4 Tetrahedral Bent CO2 G-NH3 5*-3 BF3 : CH3Cl SiF5 e;II;:÷÷÷÷÷÷÷ ClF3 T Answer key 4 0=6*6-3 §=C=:O. 2 linear linear sp N-×7=-3 µ a tetrahedral Trpicpgoanmialdae sp suis B.=3 F-IYI.SI ¥÷q÷÷ z trigonal trigonal spa G-4 ...
Valence electron worksheet lewis structures.
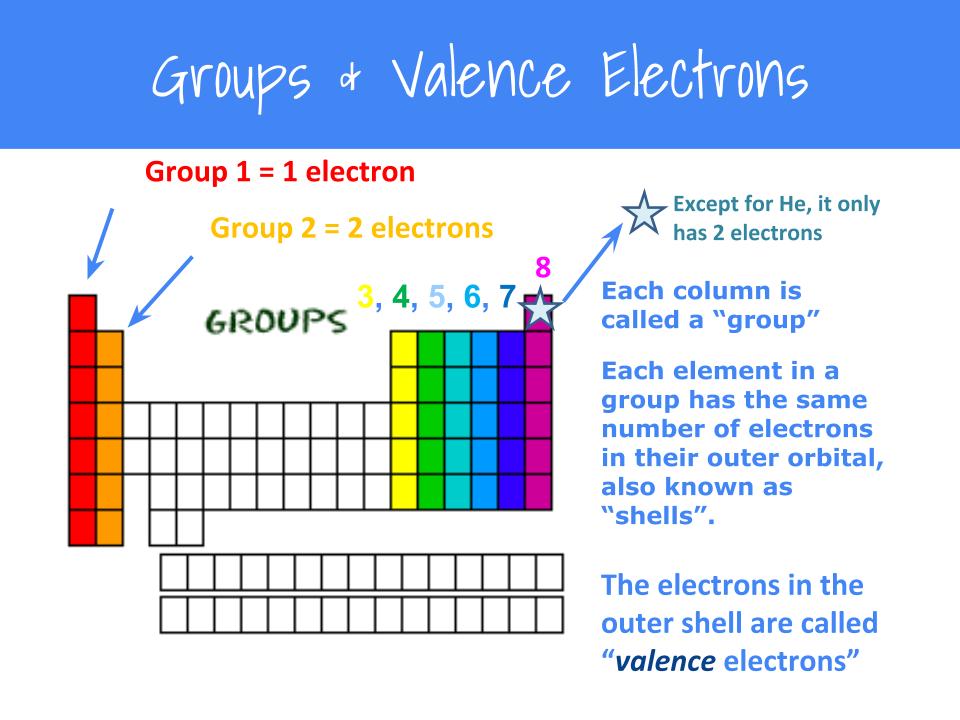
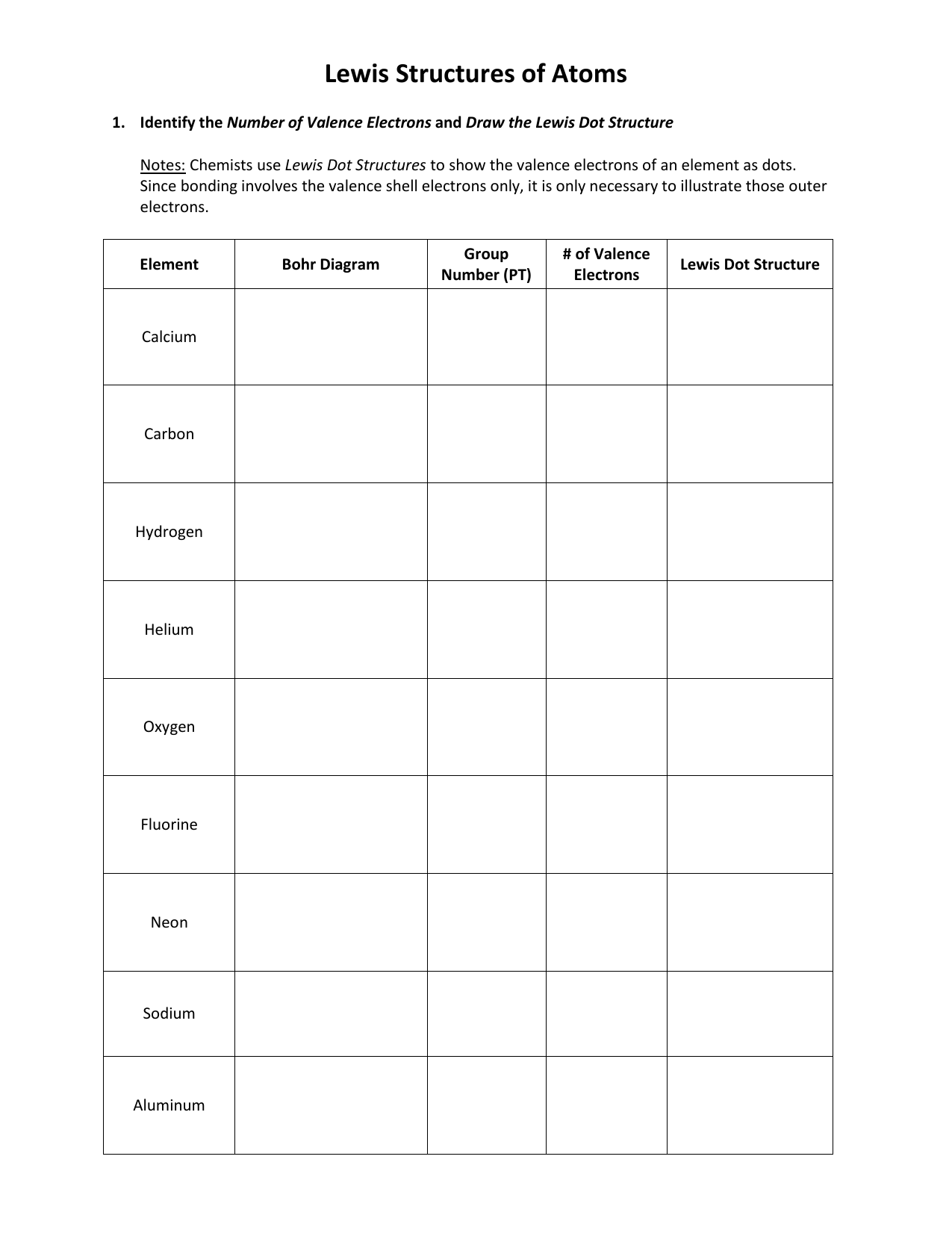
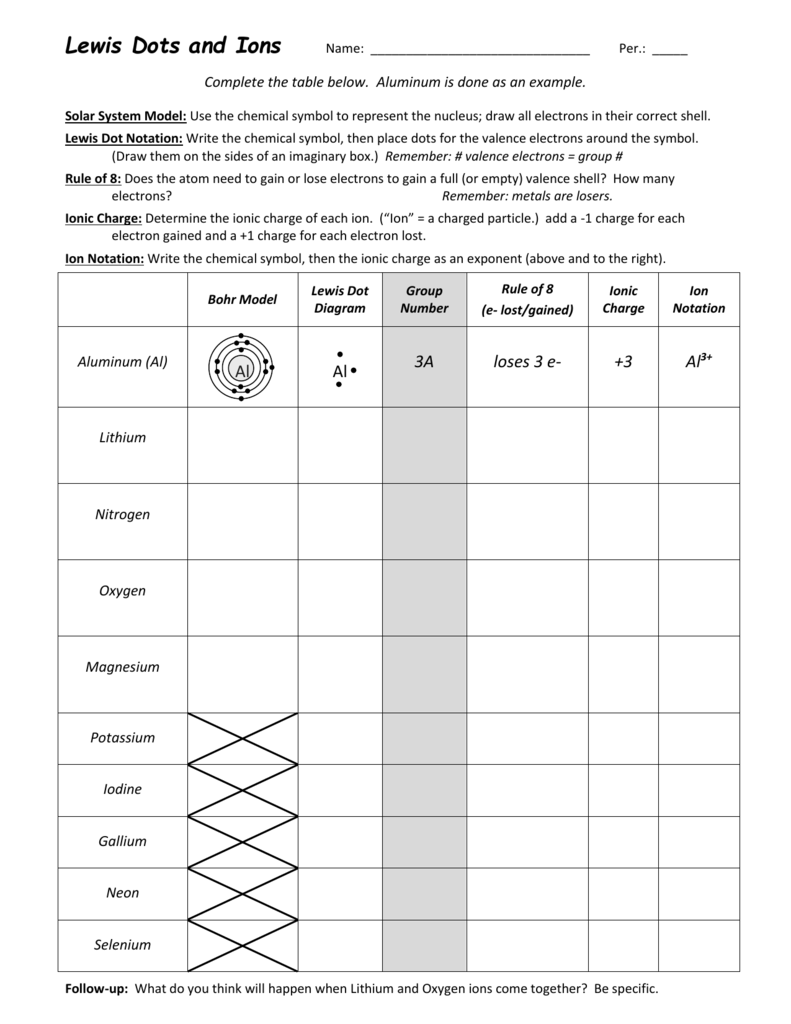
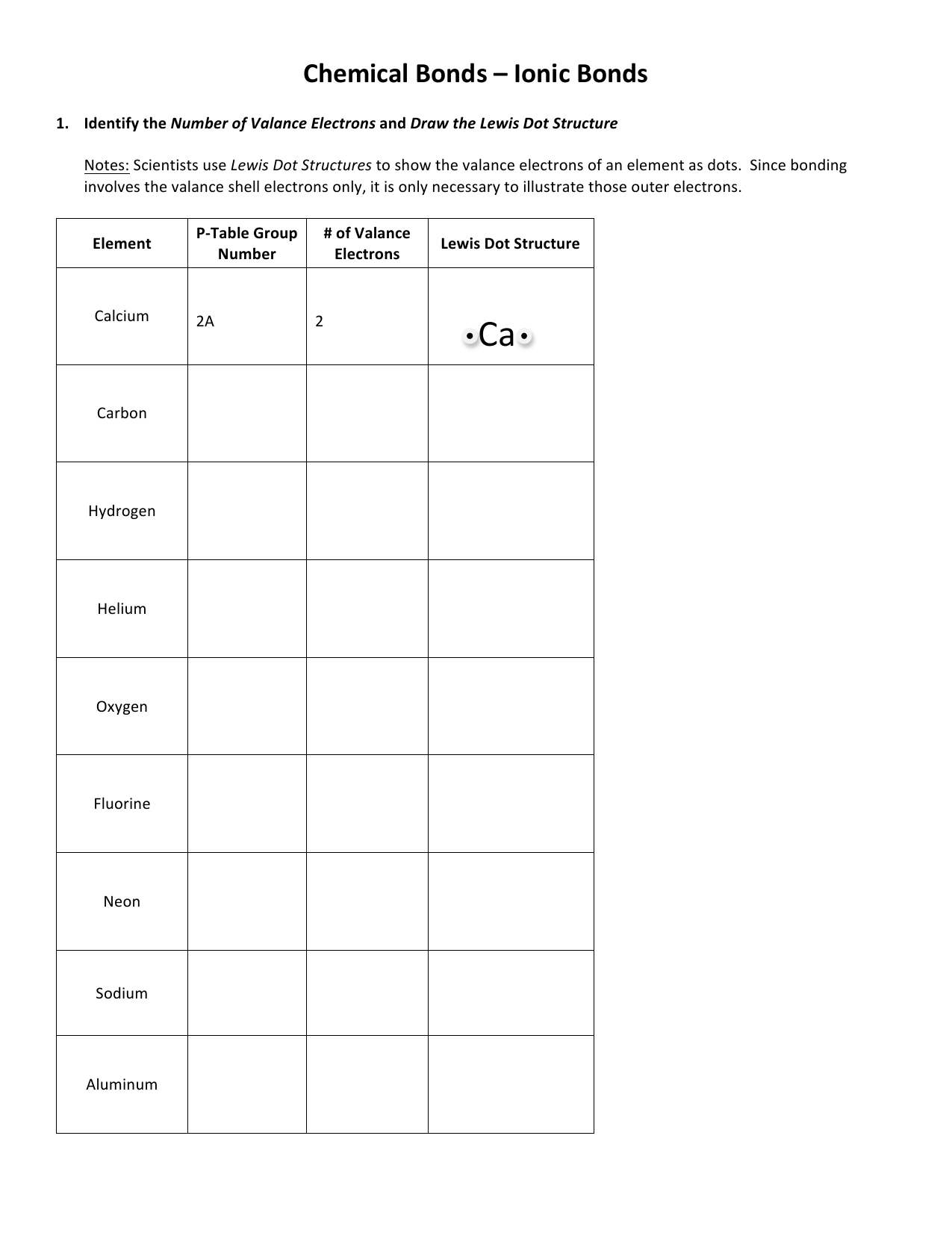
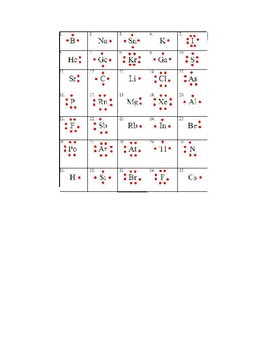
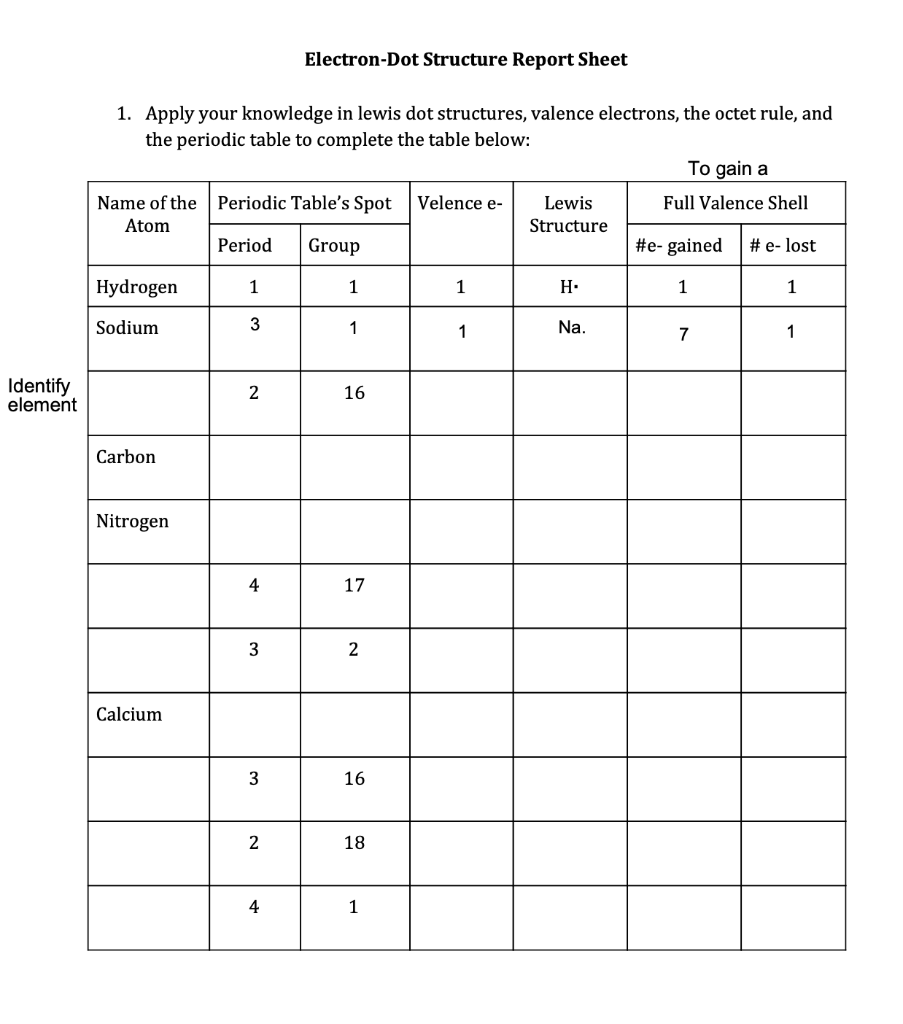
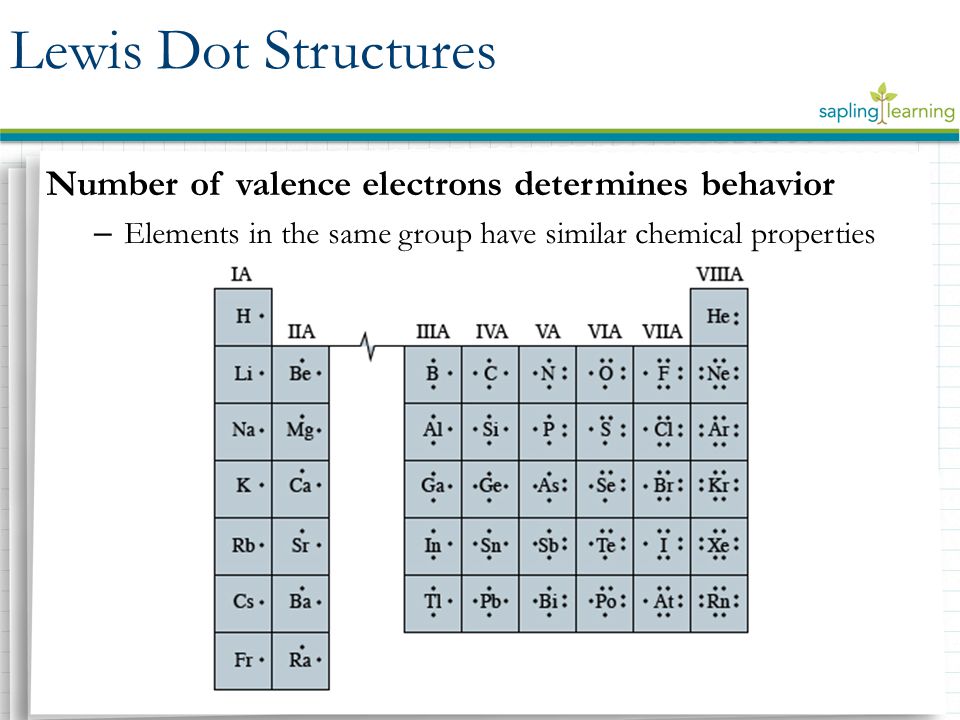
A Lewis or Electron Dot Structure is a convenient representation of the valence electrons in an atom. An electron dot structure for an atom is simply the symbol for the element, surrounded by a number of dots equal to the number of valence electrons. Avoid a common mistake: the dots represent valence electrons only, so make sure you use only ...
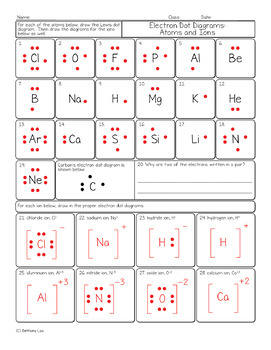
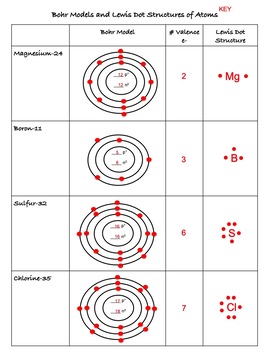
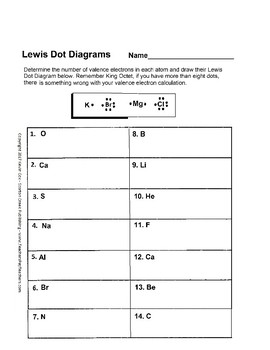
level (the valence ... Electron Dot Structure or Lewis Dot Diagram (Gilbert Lewis) A notation showing the valence electrons surrounding the atomic symbol. Lewis Structures ... Lewis Structures On your worksheet, try these elements on your own: a) H b) P c) Ca d) Ar e) Cl f) Al.
-1 determine the Lewis structure, VSEPR (electronic) geometry, hybridization, molecular shape and ideal bond angles Drawing the Lewis Structure Step 1: Determine the number of valence electrons total in the structure N: 1 x 5 = 5 and O: 2 x 6 = 12 Total = 17 but it is a negatively charged ion so we must add 1 more electron for a total of 18
PERIODIC TABLE AND VALENCE ELECTRONS WORKSHEET Author. Lewis Structures On your worksheet try. For atoms with LESS than 4valence electrons theyre going to losegive upelectrons to form positive cations. The valence electrons an element has allows us to predict its properties.























0 Response to "41 valence electron worksheet lewis structures"
Post a Comment