39 average atomic mass worksheet
August 16, 2021. August 13, 2021 By. admin. Average Atomic Mass Worksheet. Encouraged to be able to my website, in this particular period I am going to teach you in relation to Average Atomic Mass Worksheet. Why not consider photograph over? can be which remarkable???. if you think therefore, I'l l show you many image yet again beneath: Average Atomic Mass Practice Worksheet. 1. Two isotopes are known for Element X. 60.0% of all the atoms of element X weight 13.2amu. The other 40.0% of the atoms weigh 14.1amu. Find the average atomic mass of Element X. (13.2amu)(60) + (14.1amu)(40) = 13.56amu. 100. 2. Two isotopes are known for Element Y. 35.0% of all the atoms of Element Y ...
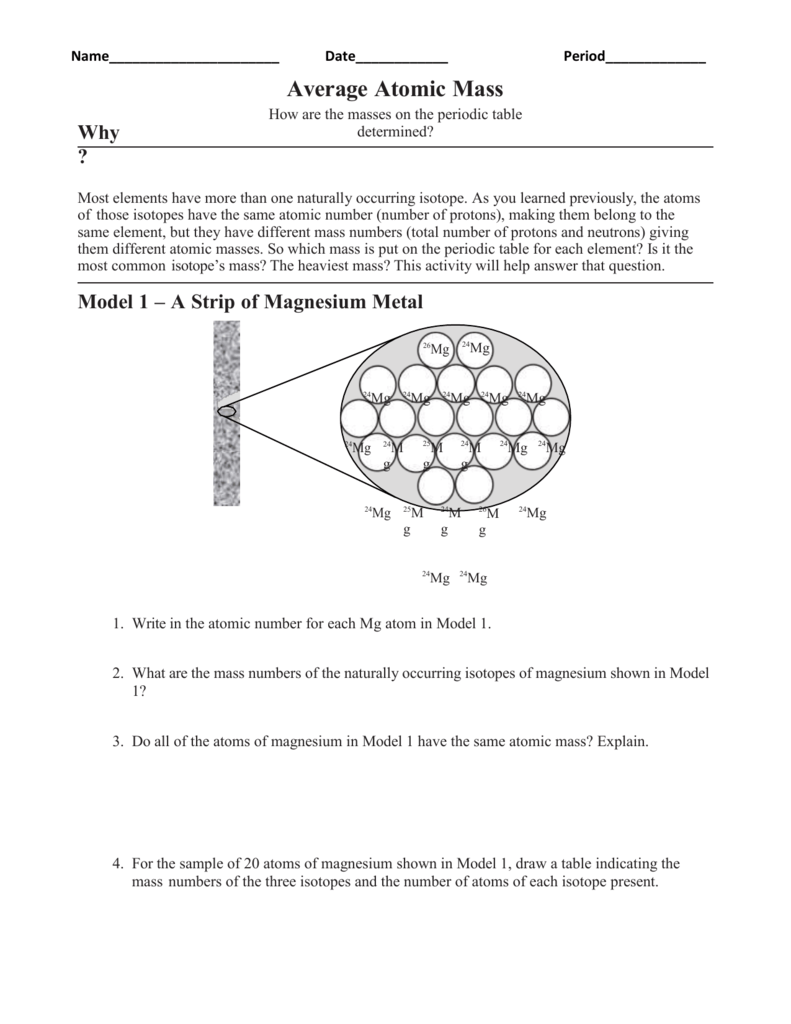
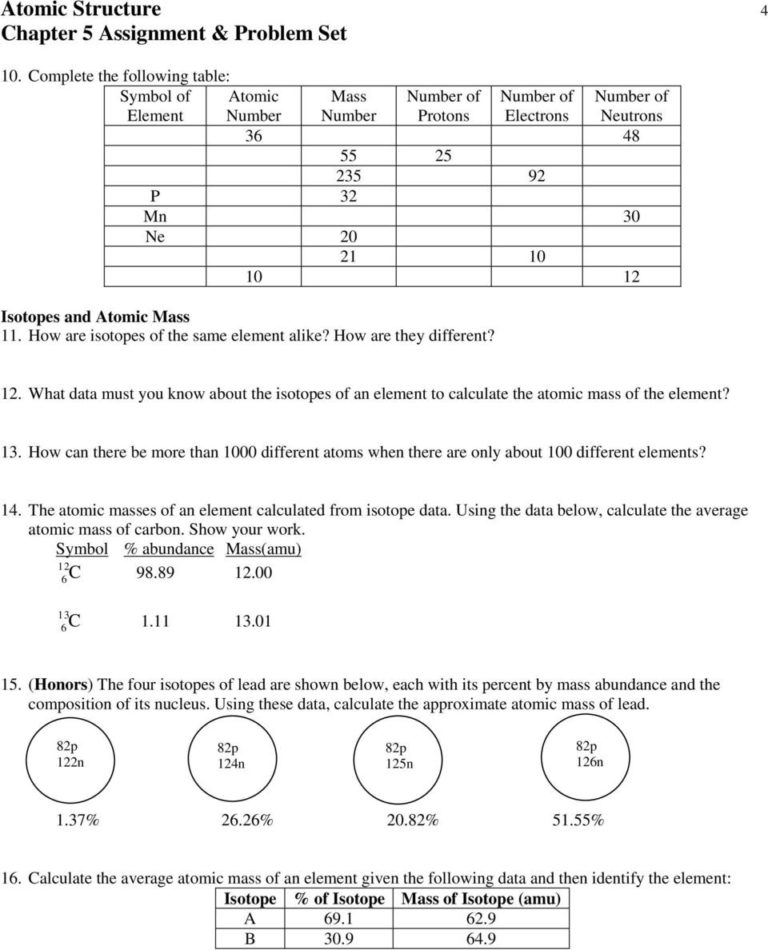
a. Which isotope has an atomic mass closest to the average atomic mass listed on the periodic table? 24.mg b. Give a mathematical reason for your answer to part a. 24 Mg is the most common isotope and is thus most heavily "weighted" in the equation for average atomic mass. 17. Boron has two naturally occurring isotopes: boron-10 and boron-11.
Average atomic mass worksheet
Complete Average Atomic Mass Worksheet 2020-2021 online with US Legal Forms. Easily fill out PDF blank, edit, and sign them. Save or instantly send your ready documents. Average Atomic Mass Worksheet: show all work. ... the abundance of 238 U is 99.28%, what is the average atomic mass of uranium? 15. Use one of the methods in Model 3 that gave the correct answer for average atomic mass to calculate the average atomic mass for oxygen. Isotope information is provided below. Show all of your work and check your answer against the mass listed on the periodic table. Isoto e Natural Abundance on Earth (0/0) Atomic Mass (am u) - 16.00 160 170 180
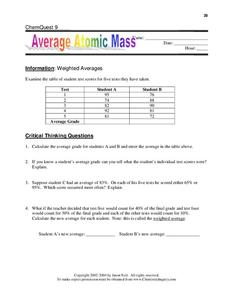
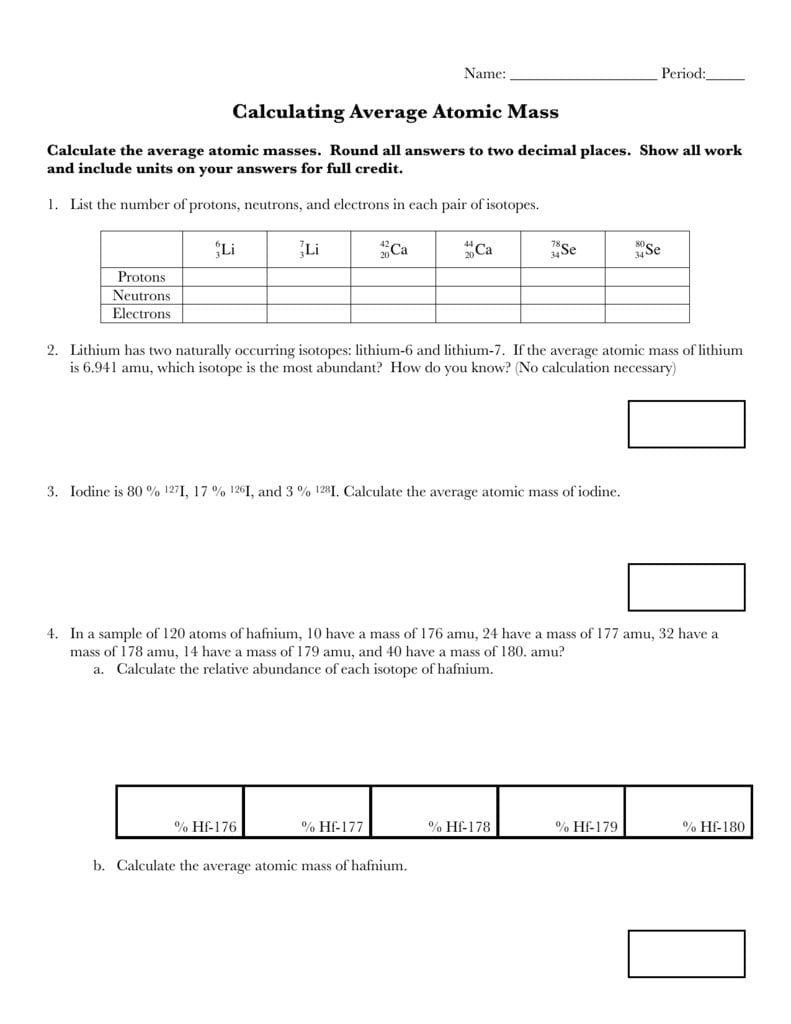
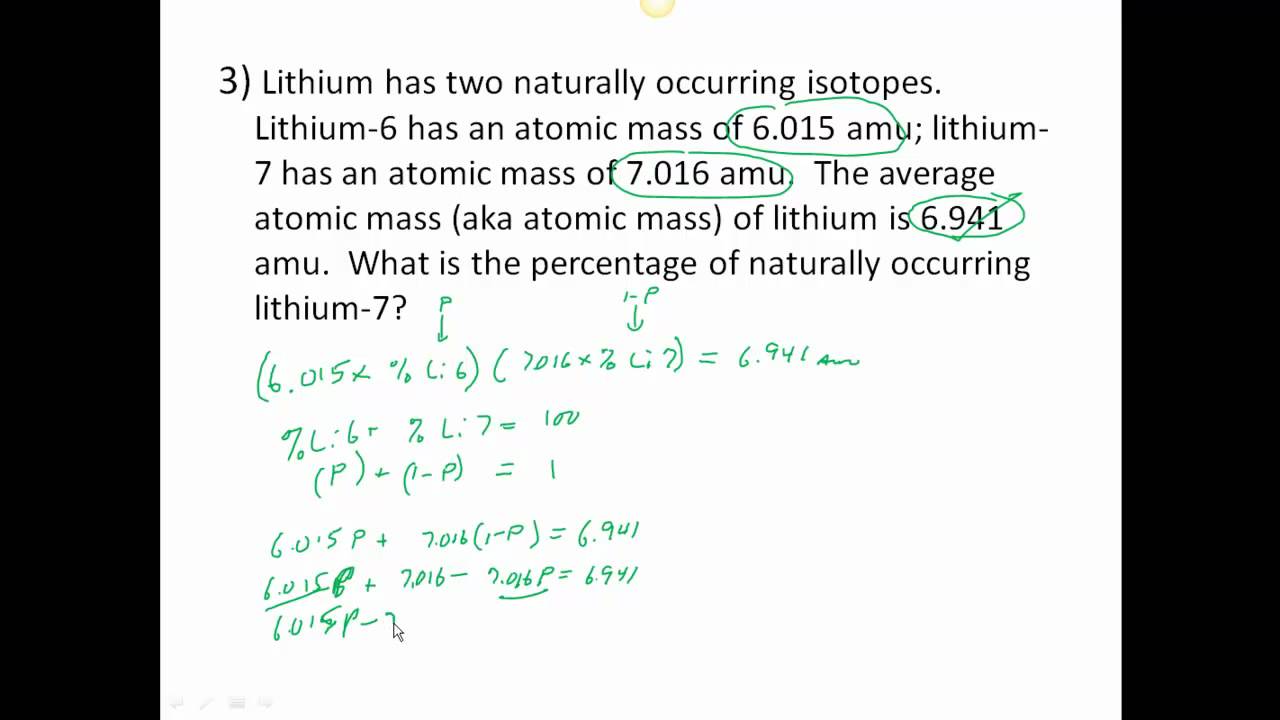
Average atomic mass worksheet. Calculate the average atomic mass of copper. 0.692( 62.93) + 0.308(64.93)=163.5 amu. 2. Calculate the average atomic mass of sulfur if 95.00 ...2 pages Isotopes and average atomic mass, as concepts, allow for the specific discussion of elements and their atoms, and this quiz/worksheet combo will help you test your understanding of these concepts. Name _____ Pd ____ Date _____ Chemistry: Average Atomic Mass Worksheet Calculate the average atomic mass for each element based on the natural abundance of its isotopes. 1. Find the average atomic mass for Li if 7.5% of Li atoms are 6 Li with a mass of 6.0151223 amu and 92.5% are 7 Li with a mass of 7.0160041 amu. Average Atomic Mass Worksheet: SHOW ALL WORK. Rubidium is a soft, silvery-white metal that has two common isotopes, 85Rb and 87Rb. The atomic mass of ...
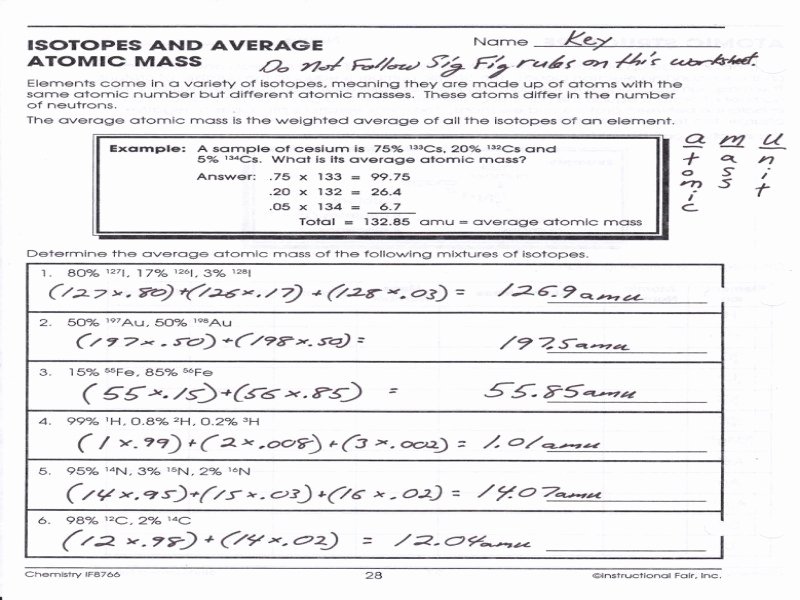
Average atomic mass worksheet solutions 1 rubidium has two common isotopes 85rb and 87rb. The average atomic mass is the weighted average of all the isotopes of an element. Weighted average of naturally occurring isotopes atomic mass 7. Worksheet May 27 2019 0328. To downloadprint click on pop-out icon or print icon to worksheet to print or ... Calculating Average Atomic Mass Calculate the average atomic mass for each of these isotopes: Isotopes of Silicon: Percent Abundance: Atomic Mass: Silicon-28 92.23% 27.97693 amu Silicon-29 4.68% 28.97649 amu Silicon-30 3.09% 29.97377 amu 1. The average atomic mass is the weighted average of all the isotopes of an element. Example: A sample of cesium is 75% 133Cs, 20% 132Cs and 5% 134Cs. What is its average atomic mass? Answer: .75 x 133 = 99.75 .20 x 132 = 26.4 .05 x 134 = Total = 132.85 amu = average atomic mass Determine the average atomic mass of the following mixtures of ... Calculate the average atomic mass of copper. 2. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 32, 0.76% has a mass of 33 and 4.22% have a mass of 34. 3. The four isotopes of lead are shown below, each with its percent by mass abundance and the composition of its nucleus.
Calculating Average Atomic Mass Worksheet 9-24-15. 30 POINTS. Each question is worth a total of 6 points: Show ALL calculation setups (worth 16/30 points), round to proper significant figures (4/30 points) and include proper units (4/30points). [NOTE - some sources of mass data do calculate the mass of electrons when determining and isotope ... Average Atomic Mass Overview The average atomic mass of an element can be determined from the relative amounts of each isotope. This is the mass used in most chemical calculations. In a naturally occurring element, the fractional abundance is the percentage of the abundance of a particular isotope in the total sample of atoms, written as a decimal. What is the average atomic mass of titanium? 47.92 amu. 4). Explain why atoms have different isotopes. In other words, how is it that.2 pages Chemistry Worksheet NAME: _____ Natural Abundance and Average Atomic Mass Block: _____ 1. Natural Abundance (NA) refers to the percentage of each isotope of an element that is found in a naturally occurring sample. Natural abundance is a percent by count - it is the number
Name _____ Calculating Average Atomic Mass Worksheet Show ALL calculation setups 1. The term "average atomic mass" is a _____average, and so is calculated differently from a "normal" average. Explain how this type of average is calculated. 2. The element copper has naturally occurring isotopes with mass numbers of 63 and 65.
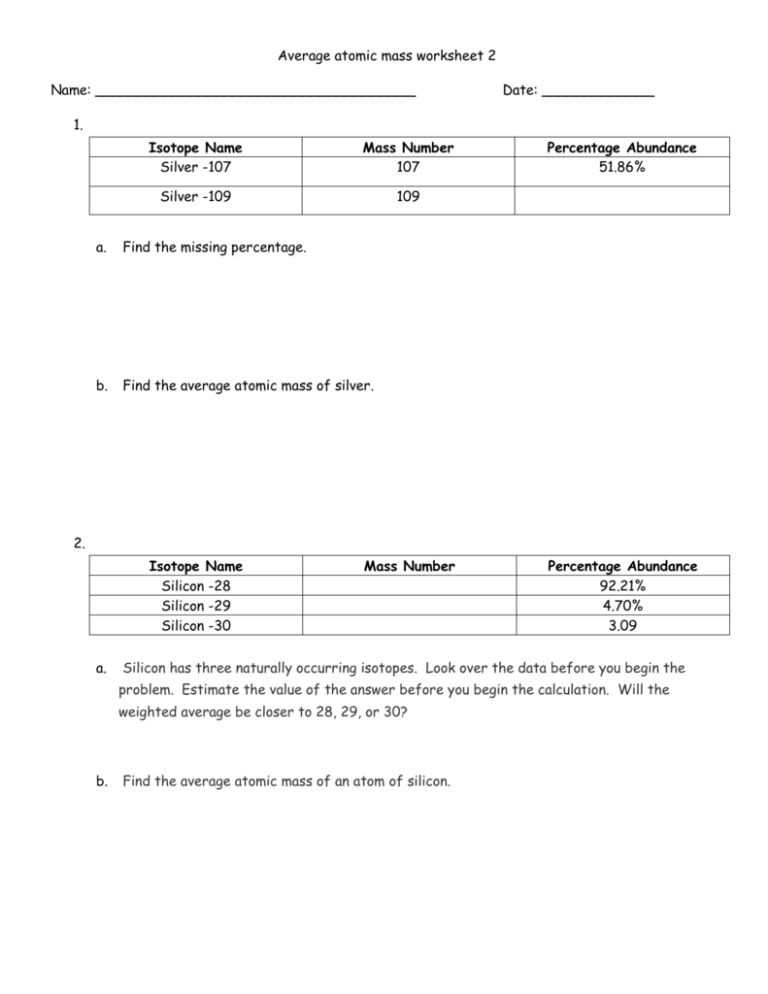
Calculating Average Atomic Mass Worksheet: 1) Three isotopes of Silicon occur in nature: Isotopes of Silicon: Percent Abundance: Atomic Mass: Silicon-28 92.23% 27.97693 amu Silicon-29 4.68% 28.97649 amu Silicon-30 3.09% 29.97377 amu Calculate the average atomic mass for the three isotopes of Silicon.
Average atomic mass worksheet. Displaying all worksheets related to average atomic mass. 75 x 133 99 75 20 x 132 26 4 05 x 134 total 132 85 amu average atomic mass determine the average atomic mass of the following mixtures of. If the abundance of 234u is 0 01 the abundance of 235u is 0 71 and the abundance of 238u is 99 28 what.
Average Atomic Mass Worksheet. by. Ms Stricklin Chemistry Corner. 4. $2.00. Word Document File. This worksheet walks students through the steps of calculating the average atomic mass of an element. Students will then practice through a couple of word problems.
Average Atomic Mass Worksheet 1) Rubidium has two common isotopes, 85Rb and 87Rb. If the abundance of 85Rb is 72.2% and the abundance of 87Rb is 27.8%, what is the average atomic mass of rubidium? 2) Uranium has three common isotopes. If the abundance of 234U is 0.01%, the abundance of 235U is 0.71%, and the abundance of 238U is 99.28%,
About This Quiz & Worksheet. Test your understanding of average atomic mass and the steps used to calculate it with this quiz/worksheet combo. All of the questions on these resources are multiple ...
Average Atomic Mass Worksheet - Worksheets are obviously the spine to students getting to know and grasping principles taught by means of the teacher. Making your individual worksheets is easy, and it lets you include simply the right material that you desire to ensure your pupils can gain knowledge of and decide to memory.
You may be offline or with limited connectivity. ... Download
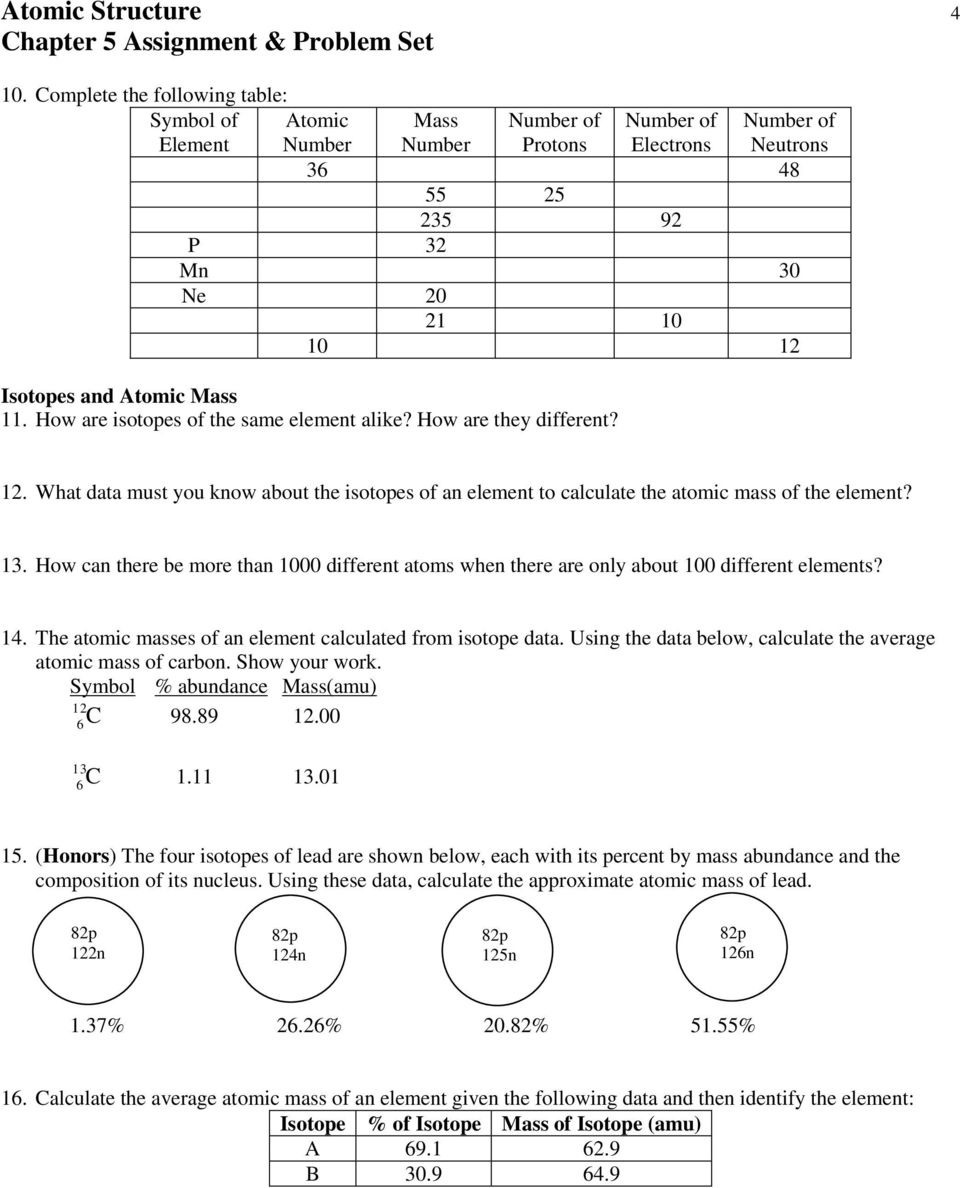
Calculate the average atomic mass of copper. 2. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.76% has a mass of 32.971amu and 4.22% have a mass of 33.967amu. 3. The three isotopes of lead are shown below, each with its percent by mass abundance and the composition of its nucleus. Using ...
An average atomic mass worksheet answers pogil in the word s authentic meaning is a piece of stationery on which one performs work. Calculate the average atomic masses. The average atomic mass of the three isotopes is 243050 amu. The average atomic mass of the three isotopes is.
Average Atomic Mass Worksheet (CP).docx ... Loading…
January 2, 2022. By. admin. Average Atomic Mass Worksheet Answers. Allowed for you to my own weblog, in this particular period I am going to show you in relation to Average Atomic Mass Worksheet Answers. Think about image previously mentioned? can be in which remarkable???. if you believe thus, I'l d show you several image once again down below:
The average atomic mass of the three isotopes is 24.3050 amu. If the atomic mass of 25Mg is 24.98584 amu, and 26Mg is 25.98259 amu, calculate the actual atomic mass of 24Mg. 8) Complete the table Isotope Mass (amu) Relative Abundance (%) Neon-20 19.992 90.51 Neon-21 20.994 Neon-22 9.22 Avg. Atomic Mass = Total %:
The Average atomic mass worksheet, on the other hand, is the number of atomic masses per unit volume of the earth's atmosphere. In the average atomic mass worksheet, the number one is the average atomic mass. The next one is the second-largest atomic mass and so on through the bottom ten.
View Average Atomic Mass Practice.docx from CHEM 456 at Orlando Christian Prep. Name: _Makayla Oates_ Period: _5th_ Average Atomic Mass Worksheet: (Show all work) 1) Rubidium is a soft, silvery-white
Average Atomic Mass Calculation Practice Worksheet .docx -... This preview shows page 1 - 2 out of 2 pages. Average Atomic Mass = Mass1 X % Isotope1 + Mass2 X % Isotope2 + (etc.) 100 Average Atomic Mass Calculation Practice Worksheet Show your work for each of the following problems. Calculation of average atomic mass is not a straight average but a weighted average based upon the percent isotopic abundance of the element.
The relative abundance and atomic masses are: 69.2% for mass of 62.93u. 30.8% fora mass of 64.93u. Calculate the average atomic mass of copper.
80.1% of the boron atoms have a mass of 11.009 amu. Calculate the atomic mass of boron. Your answer must include both a correct numerical set up and the calculated result. Base your answers to questions 2 through 4 on the following information. Isotopic notation Percent abundance Atomic mass Cu - 63 69.17% 62.930amu Cu - 65 30.83% 64.928amu
Calculating Average Atomic Mass Worksheet: 1) Three isotopes of Silicon occur in nature: Atomic Mass: 27.97693 amu 28.97649 amu 29.97377 amu Isoto es of Silicon: Silicon-28 Silicon-29 Silicon-30 Percent Abundance: 92.23% 4.68% 3.09% Calculate the average atomic mass for the three isotopes of Silicon. (-9 Y(.o ( zq.q 7377) 0/0
Pre-AP Chemistry: Worksheet #3.3 Isotopes and Average Atomic Mass 1. Name two ways that isotopes of an element differ. Mass Number, Atomic Mass, Neutrons 2. What data must you know about the isotopes of an element to calculate the atomic mass of the element? Atomic Mass of each isotope and % abundance of each isotope 3.
15. Use one of the methods in Model 3 that gave the correct answer for average atomic mass to calculate the average atomic mass for oxygen. Isotope information is provided below. Show all of your work and check your answer against the mass listed on the periodic table. Isoto e Natural Abundance on Earth (0/0) Atomic Mass (am u) - 16.00 160 170 180
Average Atomic Mass Worksheet: show all work. ... the abundance of 238 U is 99.28%, what is the average atomic mass of uranium?
Complete Average Atomic Mass Worksheet 2020-2021 online with US Legal Forms. Easily fill out PDF blank, edit, and sign them. Save or instantly send your ready documents.



























0 Response to "39 average atomic mass worksheet"
Post a Comment