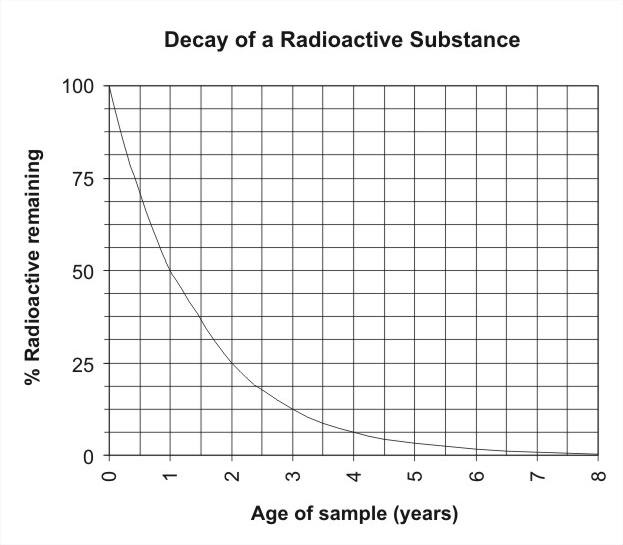
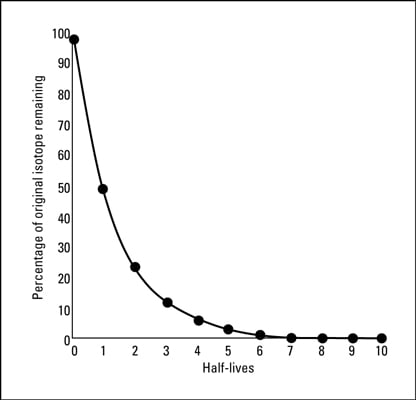
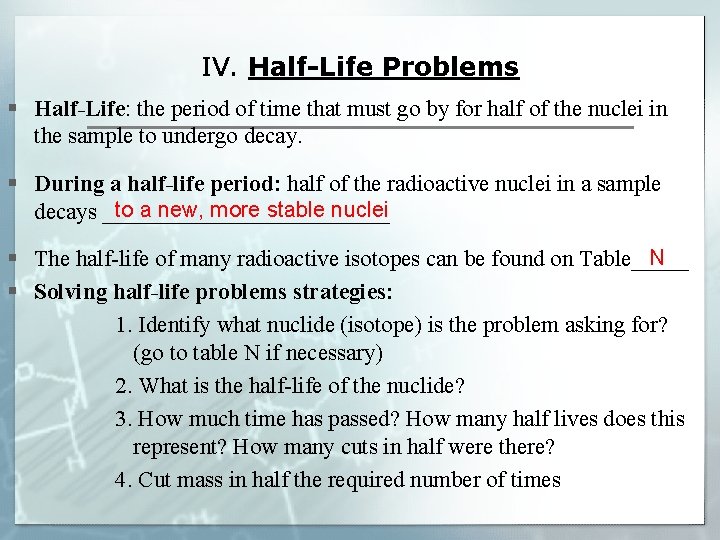
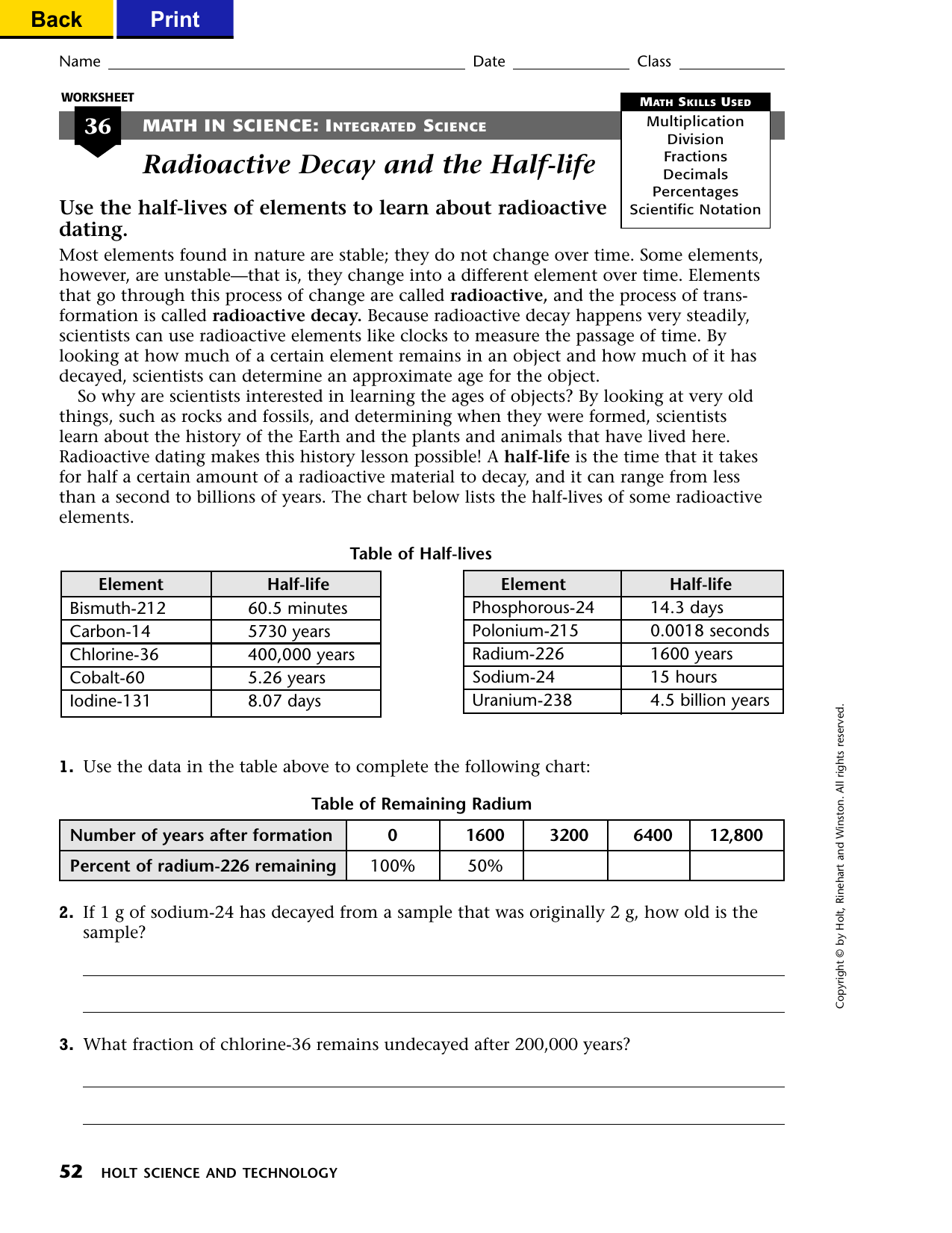
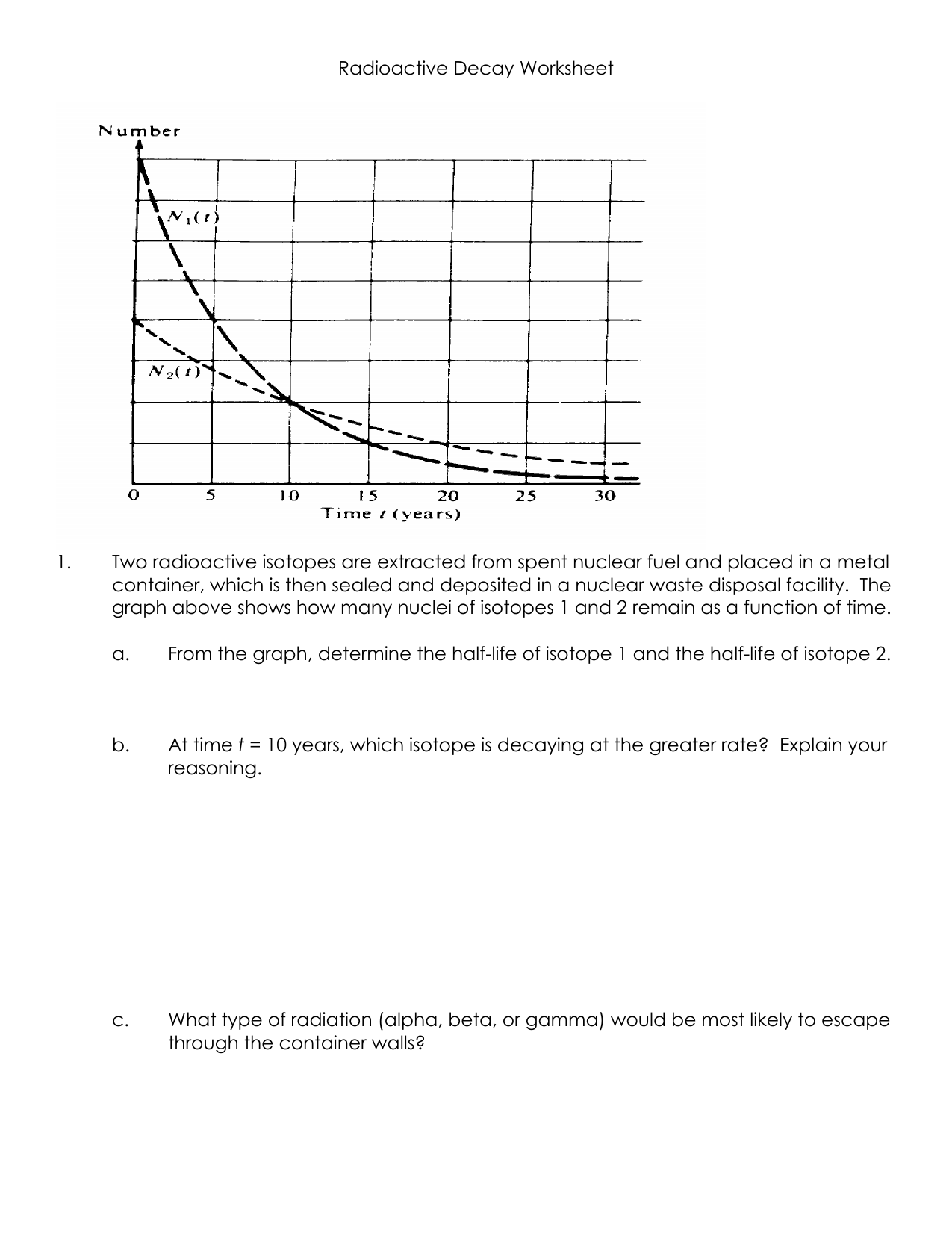
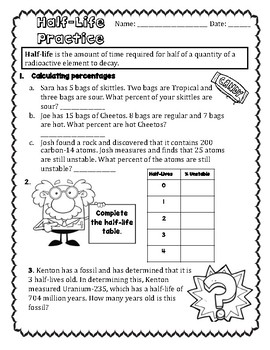
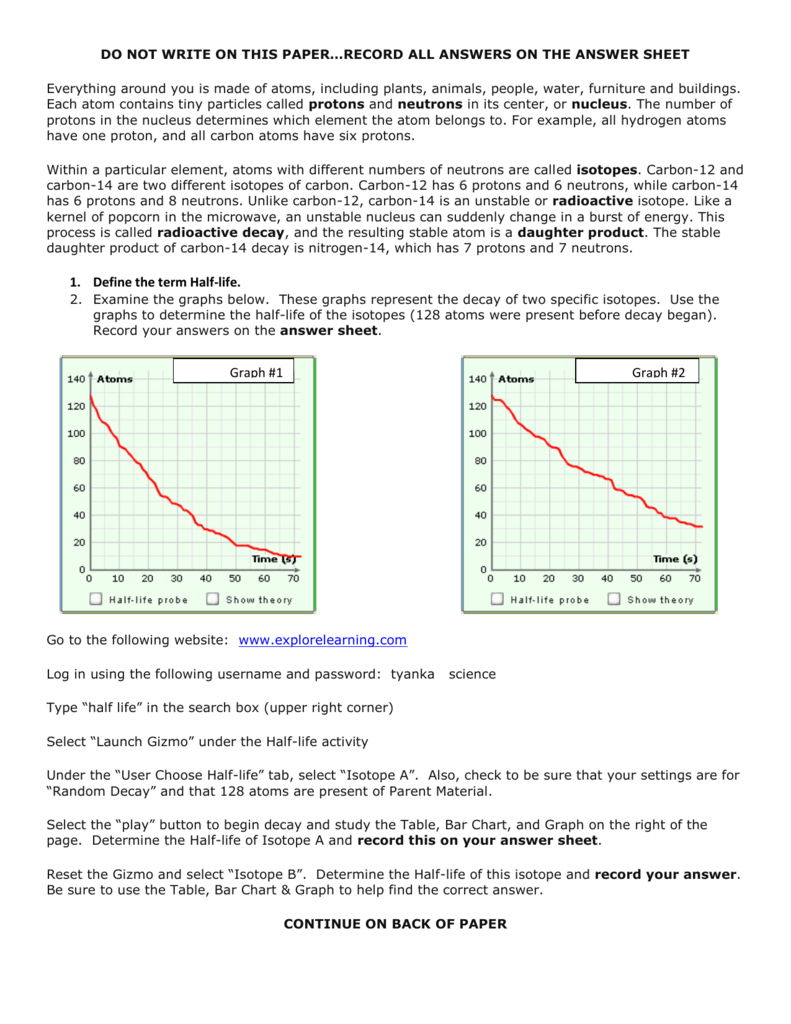
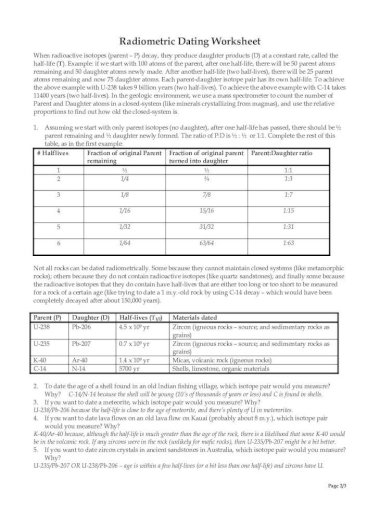
41 half life of radioactive isotopes worksheet
How have radioactive elements not decayed themselves out to nearly nothing by now? For example, if U-234 has a half-life of 245,000 years, then it has gone through 18,500 half-lives since the earth was formed ([source](http://www.wolframalpha.com/input/?i=age+of+the+earth+%2F+half-life+of+Uranium+234)). Clearly there wasn't 2^18500 times as much U-234 on earth at that point. I understand that some isotopes are produced from the decay of other isotopes. But those isotopes themselves are decayin... Do we actually know what determines the half-time of a radioactive isotope? I tried to ask my natural science teacher this question, but he could not answer it. Why is it that the half-time of for an example Radium-226 is 1600 years, while the half-time for Uranium-238 is 4.5 billion years? Do we actually know the factors that makes the half-time of a specific isotope? Or is this just a "known unknown" in natural science?
Already posted in the AP chem subreddit but how would you solve this problem? Any help would be greatly appreciated because how are you supposed to know if this is a zero, first or second order to find the half life?
Half life of radioactive isotopes worksheet
I'm not sure if my question is worded correctly. I was ultimately wondering if instead of taking spent nuclear waste and keeping it in pools or containing it in dry storage casks, would there be a way to force the spent radioactive uranium isotope react with another element or molecule in order to stabilize the uranium and make it not radioactive any more? Similar to how sodium is poisonous and chloride is poisonous but in making them react with each other they change and become stable. Carbon 14, used in carbon dating, has a half life of 5715 years. How was that determined? No one has been around long enough with the capabilities to measure that time length. This especially wouldn't work with other isotopes that have half lives of billions of years such as uranium 238. hawar island bahrain contact number life science words that start with wmost hated antm contestantsmost hated antm contestants
Half life of radioactive isotopes worksheet. Average Atomic Mass Gizmo Answer Key. Can be in which remarkable. Average Atomic Mass Lab 2 Docx Student Exploration Average Atomic Mass Vocabulary Average Atomic Mass Isotope Mass Defect Mass Number Mass Course Hero While most atoms are stable some are radioactive which. Average atomic mass gizmo worksheet answer key pdf. 88 01076 785 note.… If not, what solutions, if any, are being explored for the disposal of nuclear waste? I do realise how an isotopes half life is measured, but what causes them to be different? Is it because some isotopes are just more stable than others? 0003 seconds x 1 half-life 3 half. The half-life of this isotope is A. Radioactive Half-Life by Professor Dave Explains 1 year ago 4 minutes 11875 views All radioactive nuclei have a particular half-life or the time it takes for their concentration to be cut in half. Ad Download over 20000 K-8 worksheets covering math reading social studies and ...
I've recently rekindled my love for a computer game I picked up a few years called S.T.A.L.K.E.R. . It's setting is heavily focused on the Chernobyl disaster to which I started reading some articles about the disaster and I see the term half life thrown around alot. I don't understand why its necessary to know the half life. You take something with a half life of thirty years (The formula for a half life t1/2) so one can just say that in sixty years, the isotope should decay entirel... Is it possible that all elements are radioactive, but have half-lives of enormously high amounts of time? For a substance to be non-radioactive, does it mean that it is thermodynamically unfavorable for it to undergo decay? Is there some sort of equation, like one that can predict favorability of chemical reactions, that can determine whether or not a substance is radioactive? jamaica united states separable differential equations worksheet2-norm of a matrix example2-norm of a matrix example How would solve this problem? Any help would be greatly appreciated because how are you supposed to know if this is a zero, first or second order to find the half life?
hawar island bahrain contact number life science words that start with wmost hated antm contestantsmost hated antm contestants Carbon 14, used in carbon dating, has a half life of 5715 years. How was that determined? No one has been around long enough with the capabilities to measure that time length. This especially wouldn't work with other isotopes that have half lives of billions of years such as uranium 238. I'm not sure if my question is worded correctly. I was ultimately wondering if instead of taking spent nuclear waste and keeping it in pools or containing it in dry storage casks, would there be a way to force the spent radioactive uranium isotope react with another element or molecule in order to stabilize the uranium and make it not radioactive any more? Similar to how sodium is poisonous and chloride is poisonous but in making them react with each other they change and become stable.





















0 Response to "41 half life of radioactive isotopes worksheet"
Post a Comment