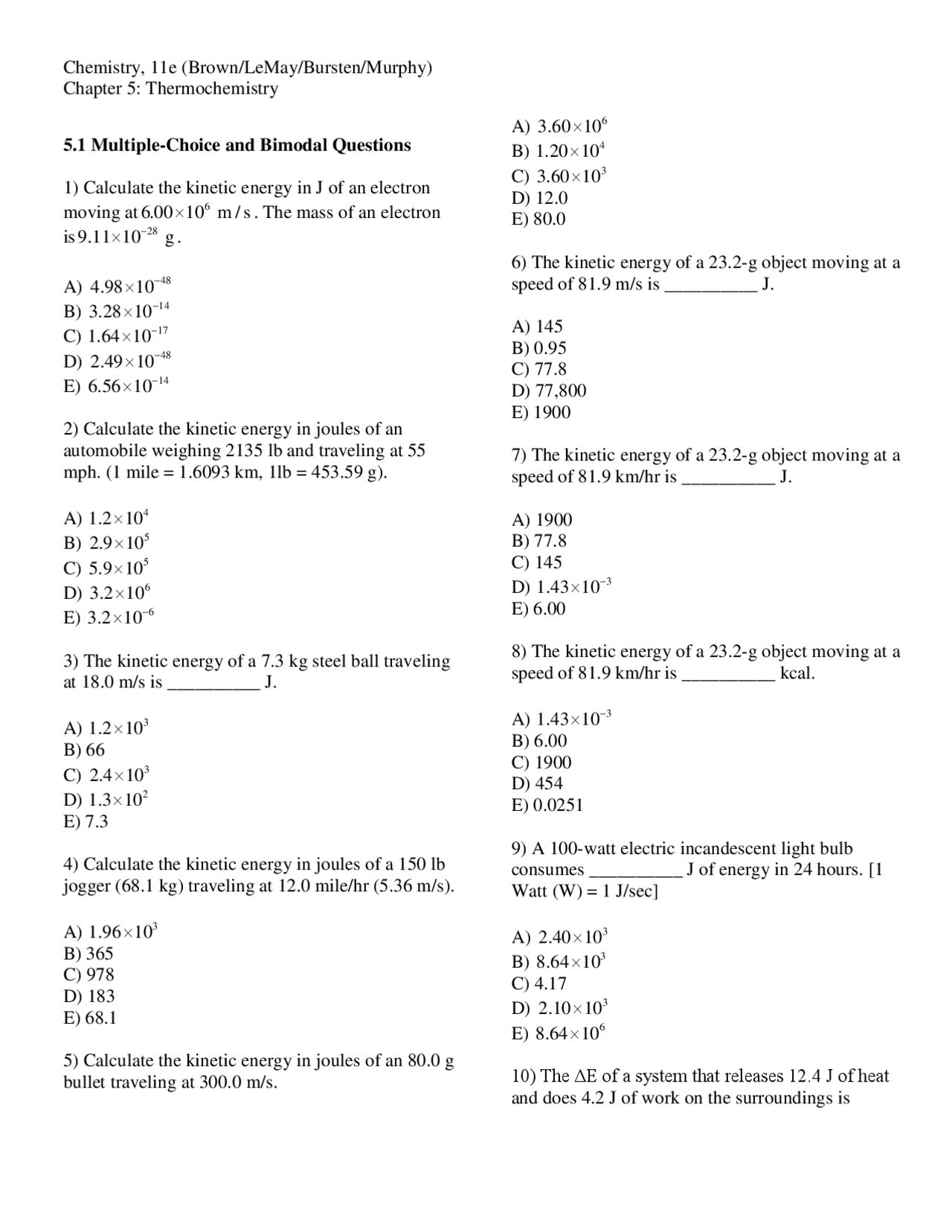
42 thermochemistry problems worksheet number one answers
March 3, 2021 - In addition to mass changes, chemical reactions involve heat changes associated with changes in the substances’ internal energy. Like mass-based stoichiometry, these changes are quantitative. … Academia.edu is a platform for academics to share research papers.
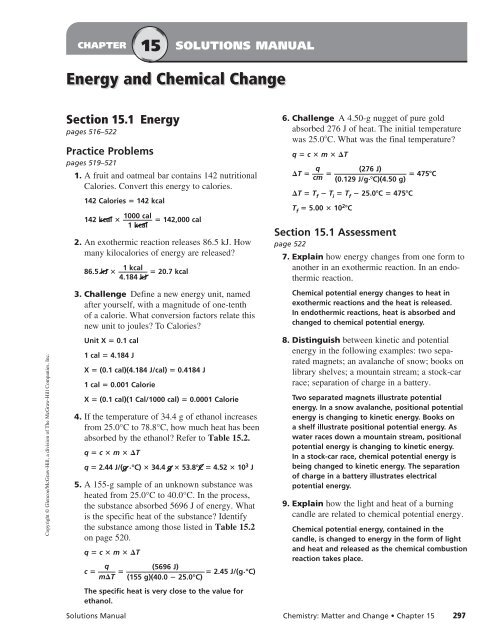
Dec 17, 2021 · Standard heats of formation worksheet answers 1. Worksheet – Thermochemistry – AP level Back to the Thermochemistry Menu Problem 1. Practice file answer key. 2 2 267g 078345 mol H S 3408 gmol H S so 406 kJ was released when 078345 moles of H 2 S reacted. Free Thermochemistry Worksheets.

Thermochemistry problems worksheet number one answers
Thermochemistry Answers - Worksheet Number One. We will ignore any heats losses to the walls of the container and losses to the air. These is a typical position to take since, in a real experiment, both would have to be accounted for, making for much more complexity. 1. q = (20.0 g) (20.0 °C) (2.02 J/g °C). (Note C p of gas is used.) Free essays, homework help, flashcards, research papers, book reports, term papers, history, science, politics One side of a rectangle is 7 inches and another is 9 inches. What is the perimeter of the rectangle in inches? Possible Answers: Correct answer: Explanation: To find the perimeter of a rectangle, add the lengths of the rectangle's four sides. If you have only the width and the height, then you can easily find all four sides (two sides are each equal to the height and the other two sides are ...
Thermochemistry problems worksheet number one answers. Practice Problems on Working with Grams, Moles, Avogadro's number, Stoichiometry (Requires Adobe Acrobat Reader) ... Answers to Practice Problems on Working with g, mol, Avogadro's number, Stoichiometry(Requires Adobe Acrobat Reader) Nous voudrions effectuer une description ici mais le site que vous consultez ne nous en laisse pas la possibilité. February 7, 2021 - Chemistry is the science of matter, especially its properties, structure, composition, behavior, reactions, interactions and the changes it undergoes. Chemistry is sometimes called "the central science" because it connects physics with other natural sciences such as astronomy, geology and biology. July 27, 2015 - Thermochemistry Problems Worksheet One Semester - Free download as PDF File (.pdf) or read online for free. Thermochemistry Problems-worksheet, All the best
Assume that no heat is lost during the process. Answer: 40. °C. Analysis of the situation: What happened: 328 g of water went from 32.4 °C to. CHM 150/151 Student Resources · If something is missing, let Dr. Bob know Thermochemistry Problems - Worksheet Number One (answers available on web site) 1. How much energy must be absorbed by 20.0 g of water to increase its ... Thermochemistry Answers - Worksheet Number One We will ignore any heats losses to the walls of the container and losses to the air. These is a typical position to take since, in a real experiment, both would have to be accounted for, making for much more complexity. 1. q = (20.0 g) (20.0 ツーC) (2.02 J/g ツーC)= 808 J.
Bond Number of Bonds/Coefficient Bond Energy (each) Total Energy H 2 2 432 864 O 2 1 204 204 Total 1,068 kJ Products Bond Number of Bonds/Coefficient Bond Energy (each) Total Energy OH 4 467 1,868 Total 1,868 kJ 1,068 kJ – 1,868 kJ= -800 kJ = Exothermic Note: You should recognize this problem from the end of the video. The Brookville Schools Safe Return to In-Person Instruction & Continuity of Services Plan can be found here. The district is receiving ARP ESSER funds. More Thermochemistry with answers displaying top 8 worksheets found for this concept. 2nh 3 22kcal n 2 3h 2 h 2. Chem 121 extra practice problems for thermochemistry spring 2006 these problems are not meant to introduce the problems associated with thermochemistry. 2f 2 g 2h 2 o l 4hf g o 2 g h. Specific Heat, Bomb Calorimeter, Enthalpy, Thermochemical Equations, High School Chemistry. examples and step by step solutions
Calculate the standard enthalpy of reaction for the process nh 3 g hcl g nh 4 cl s h. Download file pdf thermochemistry worksheet 1 answers thermochemistry problems worksheet number one thermochemistry exam1 and problem solutions. We will ignore any heats losses to the walls of the container and losses to the air. Na 2 o s so.
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds composed of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances.. In the scope of its subject, chemistry occupies an …
One or more sample assessments. One or more follow-ups/extensions. A list of resources . Sample released SOL test items for each Organizing Topic. School divisions and teachers can use the Enhanced Scope and Sequence as a resource for developing sound curricular and instructional programs. These materials are intended as examples of ways the essential …
thermochemistry problems worksheet number one answers is available in our book collection an online access to it is set as public so you can get it instantly. Our digital library hosts in multiple locations, allowing you to get the most less latency time to download any of our books like this one.
View Notes - Thermochemistry Problems Worksheet One - Key from SCIENCE Parallel C at Central Bucks High School South. KEY Thermochemistry Problems — Worksheet Number One (answers available on web
1) Analyze – List the knowns & the unknown. Use dimensional analysis to determine the mass of the water. You must also calculate ΔT. Use ΔH= -q surr = - mcΔT to solve for ΔH. 2) Calculate – Solve for the unknown. c = 4.184 J/g⁰C V final = V HCl + V NaOH = 25.0 mL + 25.0 mL = 50.0 mL T i = 25.0⁰C T f = 32.0⁰C Density solution = 1 ...
General Information Advice for adding my class Letter of Recommendation Links to Classwork Syllabus, Calendar, and other first week papers of importance Web Assignment 1 Weekly agendas Lecture handouts by Chapter Labs Manual and Experiment Information Practice exams, answer keys Teaching Schedule ...
Thermochemistry answers worksheet number one. In this lesson we will learn. The study of heat changes that accompany chemical reactions and phase changes learn with flashcards games and more for free. A energy can only be transferred as potentlal energy being converted to kinetic energy.
Number of pages. Problems are on first law and enthalpy with answers. Preview the document. Uploaded on 06/11/2021. hambery . 3 reviews - 41 documents.
Reaction rate is the number (mol) of molecules produced or consumedi h i l ti ti l (d in a chemical reaction per reaction volume (L)ti() per time (s) rate forward rate 29 forward. Origin of Equilibrium Constant For simple reactions (like this one), reaction rate is proportional to the concentrations of the reactants raised to their stoichiometric coefficients Rate definition: rate forward rate ...
What is the total number of electrons that can occupy the principal quantum level n = 4 in one unit_outline-energy_changes_and_rates_new_2013. 412-416. Unit 3 – Energy Changes and Rates of Reaction. 1 Functional Groups 10 #1-4, #1-3 1. Run starting. 8): Wed. start Unit 4 May 8, 2017: Unit 3 Test Ontario Virtual School 1 SCH4U Ms. Study Resources . 9): Thurs. 3 reactions …
By using this site you agree to the use of cookies for analytics, personalized content and ads. Learn more · Register for a FREE Schoolnotes account and create pages for posting homework, creating flashcards, and sharing information with your parents and students
Chemical engineering design - GAVIN TOWLER, RAY SINNOTT.pdf
Thermochemistry Problems – Worksheet Number One Author. Answers worksheet number one we will ignore any heats losses to the walls of the container and losses to the air. USER Last modified by. 1 A chemical reaction that absorbs heat from the surroundings is said to be _____ and has a _____ DH at constant pressure.
Thermochemistry Problems - Worksheet Number One (answers available on web site). 1. How much energy must be absorbed by 20.0 g of water to increase its ...
Answers, Thermochemistry Practice Problems 2 1 6. When 26.7 g of H 2 S was burned in excess oxygen, 406 kJ was released. What is H for the following equation? 2 H 2 S(g) + 3 O 2 (g) 2 SO 2 (g) + 2 H 2 O(g); H = ??? Answer: -1040 kJ Reasoning: 2 2 26.7g 0.78345 mol H S 34.08 g/mol H S, so 406 kJ was released when 0.78345 moles of H 2 S reacted. 2 2
Chemistry unit 7 supplement to worksheet 4 answer key Chem worksheet 16 1 answers. Lab 16-4 Heat of Solution. 1, Table E10 . 4 23. Specific heat chem worksheet 16 1 answer key. 0'. " The 16:1 ratio will always be true as long as the number of eggs is the same. Answers to Thermochemistry Worksheets Heat Worksheet 1. -20.7 kcal 2. 7.0.0612 kcal 3.
One side of a rectangle is 7 inches and another is 9 inches. What is the perimeter of the rectangle in inches? Possible Answers: Correct answer: Explanation: To find the perimeter of a rectangle, add the lengths of the rectangle's four sides. If you have only the width and the height, then you can easily find all four sides (two sides are each equal to the height and the other two sides are ...
Free essays, homework help, flashcards, research papers, book reports, term papers, history, science, politics
Thermochemistry Answers - Worksheet Number One. We will ignore any heats losses to the walls of the container and losses to the air. These is a typical position to take since, in a real experiment, both would have to be accounted for, making for much more complexity. 1. q = (20.0 g) (20.0 °C) (2.02 J/g °C). (Note C p of gas is used.)






























0 Response to "42 thermochemistry problems worksheet number one answers"
Post a Comment