45 emission spectra and energy levels worksheet
PPTX PowerPoint Presentation A quantum of energy in the form of light is emitted when the electron drops back to a lower energy level. 5.3 An Explanation of Atomic Spectra The light emitted by an electron moving from a higher to a lower energy level has a frequency directly proportional to the energy change of the electron. 5.3 An Explanation of Atomic Spectra Chemistry 101 8-ATOMIC EMISSION SPECTRA excited absorb ... Chemistry 101 8-ATOMIC EMISSION SPECTRA. Knowledge of the arrangement of electrons around the nuclei of atoms has been obtained by examining the light emitted by excited atoms.Atoms become excited when they absorb energy; they then emit energy in the form of light as they return to a less excited state.Under
filmreview.us A mass spectrum of Atomic Spectra Worksheet Key Directions In this exercise you will be given some terminology to define. 001. 3 atomic emission spectra worksheet answers, interpret emission spectra worksheet answers, atomic emission spectra worksheet answers, emission spectra and energy levels worksheet answers, worksheet emission and ...

Emission spectra and energy levels worksheet
Electron Energy Level Doodle Teaching Resources | TpT This activity worksheet, reviews the emission spectrum of hydrogen including the electromagnetic spectrum, where students identify frequency, energy and wavelength. Labeling and describing absorption spectra and emission spectra, including a continuous spectrum, to identify the differences between them. PDF Emission Spectra - Michigan State University Emission Spectra Data Collection and Analysis Do the following steps for each light source (5 in total) after you've set it up: 1. Observe the light source through the diffraction grating. You may need to hold the grating at an angle to observe the interference pattern. Spectra and energy levels | Teaching Resources A series of discussions and demonstrations looking at spectra and emission levels. Tes classic free licence. Report this resource to let us know if it violates our terms and conditions. Our customer service team will review your report and will be in touch. £0.00.
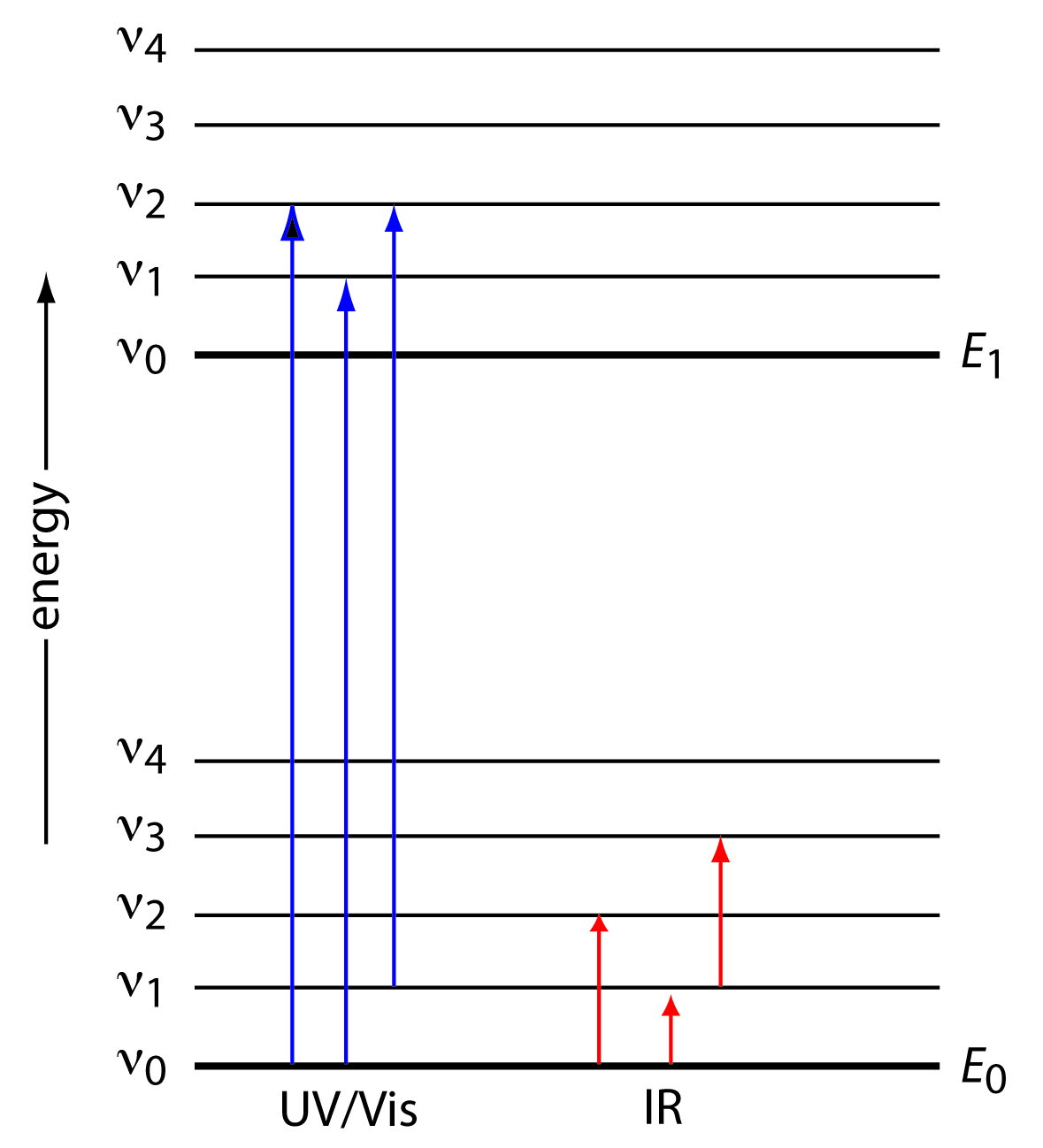
Emission spectra and energy levels worksheet. Atomic Spectrum: Definition, Absorption & Emission - Video ... Oct 11, 2021 · Learn the definition of the atomic spectra, the function of emission spectra. and the role absorption spectra play in the visibility of colors like those seen in a … PDF Atomic Emission Spectra Atomic Emission Spectra The electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. The ground state of an atom is the lowest energy state of the atom. When those atoms are given energy, the electrons absorb the energy and move to a higher energy level. Types of Spectra Flashcards | Quizlet Types of Spectra. continuous, emission, absorption. Continuous spectrum. solid or liquid gas heated to incandescence - almost perfect. "Hot dense light source" "core of a star". emission spectrum. black background, single band of light. warm or hot electrons, will only see the colors of light emitted by the atoms. wiens law. Analyzing Spectra - Weber State University An emission spectrum involves transitions of electrons from _____ to _____ energy states. An absorption spectrum involves transitions of electrons from _____ to _____ states. These transitions occur only between discrete energy levels, and thus the lines occur only at certain wavelengths and at no others.
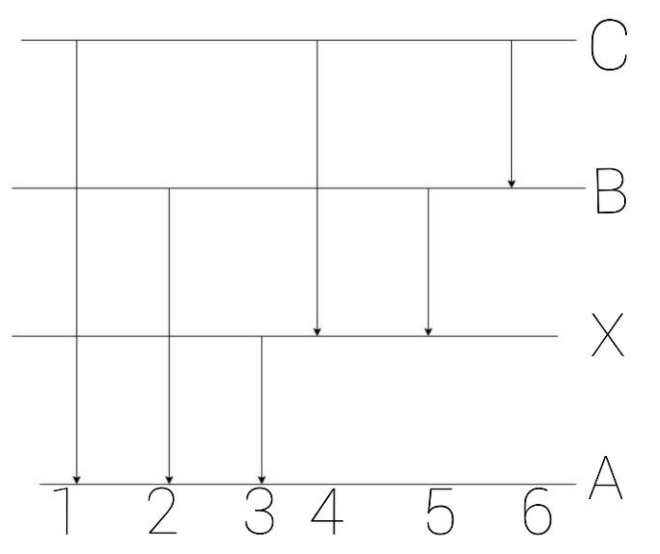
DOC Astronomy Worksheet Emission lines are spikes above the continuum, while absorption lines are dips or spikes below the continuum. Each emission or absorption line is the result of electron transitions between energy levels in atoms or molecules. Whether it is emission or absorption just depends on its state: Hot gases = emission, cool gases = absorption. Energy Levels and Spectra - Physics Things Calculate the energy difference (in Joules) between energy levels. Using either E = hf, E = hc/wavelength or for electrons E = 1/2mv^2 you can work out the energy of the incident photon/electron. If this energy is equal to the difference in energy levels, the electron will be excited. Spectra Of Elements Worksheet Answers The emission spectrum consists of discrete lines corresponding to the differences in energy levels characteristic of and unique along the atoms of the element. Emission Spectra of 10 Elements Answer Key p5 Have students examine the. Photon of radiation that shows up bow this range line-emission spectrum. Worksheet 1 contains the spectra for 7 ... Pre-lab: Emission Spectra and Energy Levels You'll ... Start studying Pre-lab: Emission Spectra and Energy Levels. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Vibrational energy chart - benandmarina.us email protected] Pearson physics 30 chapter 14 solutions - veronikakocianova.de email protected] [email protected] [email protected] 0 km/h. The NCERT Solutions for Class 7 Science is one of the most crucial resources to refer to excel in Class 7 Science. tw on October 11, 2021 by guest Physics: A Strategic Approach Plus Mastering Physics with Pearson eText -- Access Card Package Package consists of: 0134609034 / 9780134609034 Filed Under: … Solved Atomic Emission Spectra Worksheet Part I: | Chegg.com Atomic Emission Spectra Worksheet Part I: Calculations of Electron Energy Levels (E.) in the Bohr Atom of Hydrogen bebe Each shell in the Bohr atom has a positive integer value "n" that represents an energy level for that orbit. rvpconsultant.us Oct 28, 2014 · Unit 2 worksheet 3 physics answers. email protected] [email protected]
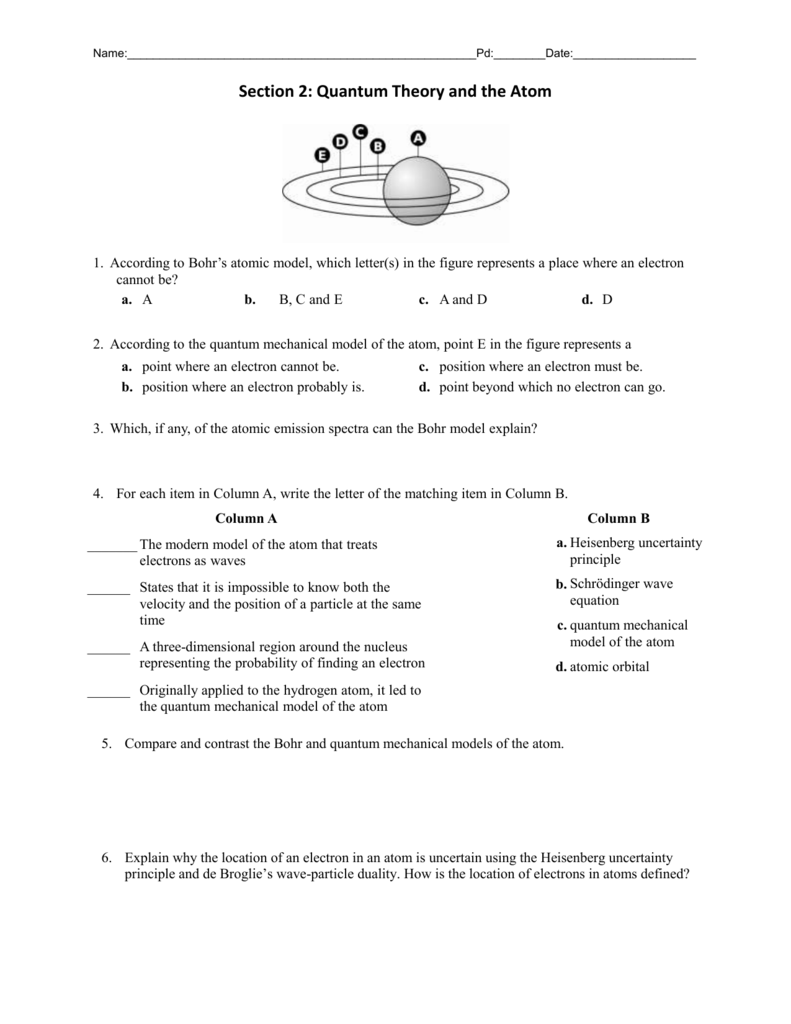
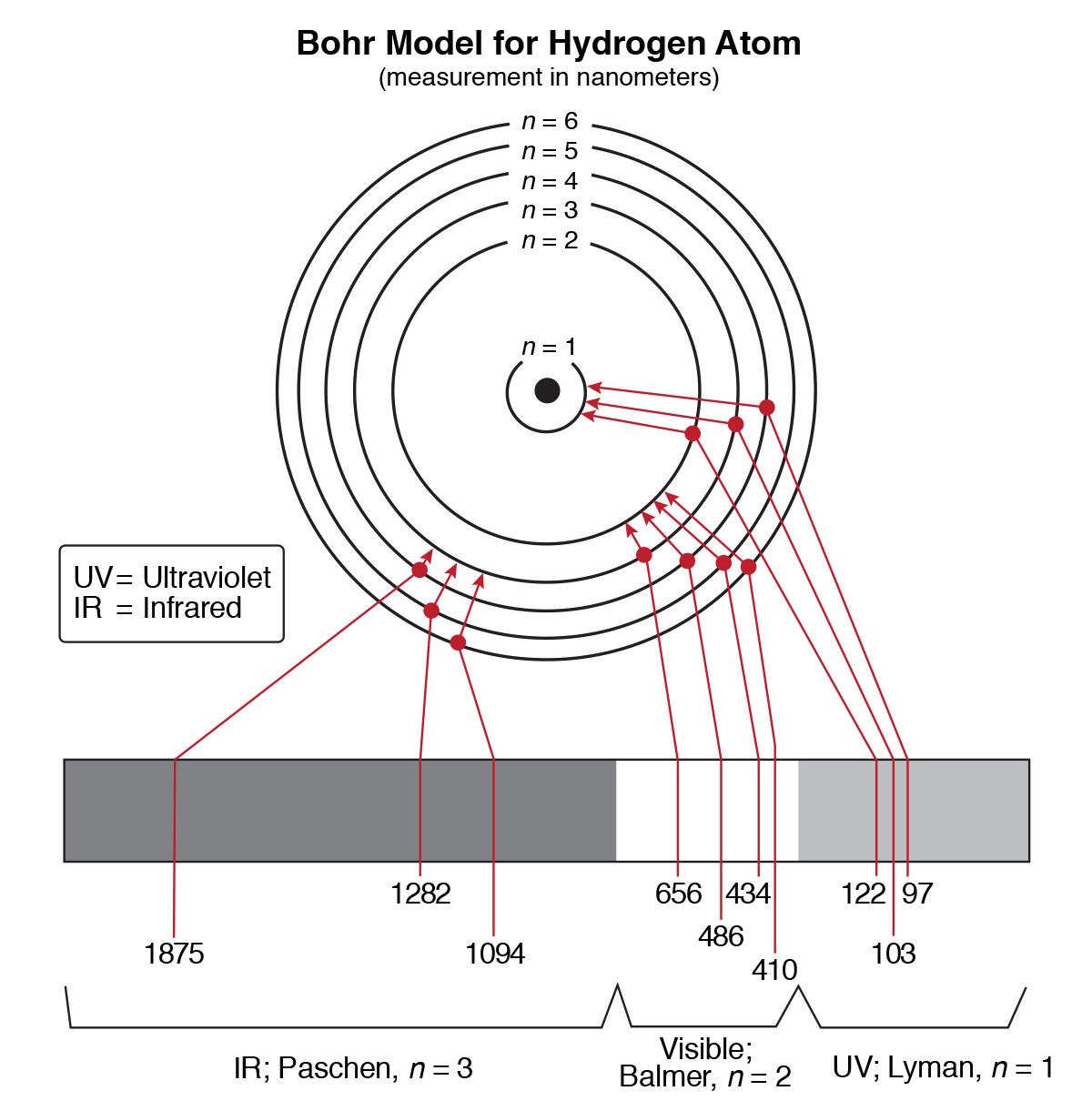
PDF Worksheet 1.3 Emission spectra and electron configurations No. Question Answer 1In which region of the electromagnetic spectrum would you find lines of the greatest energy—ultraviolet, visible or infrared? 2On the energy level diagram show the electron transitions that would result in the series of lines that are seen in the visible region of the hydrogen emission spectrum.
PDF X-ray Spectroscopy and the Chemistry of Supernova Remnants ... Student Worksheet: Graphing Spectra Part 2 The following spectrum represents the energy state of the element, carbon. Carbon's emission lines in the visible range are a function of wavelength from 4,000 to 7,000 Ångstroms. You are going to create a graphical representation of carbon's spectrum from the photographic representation.
2 Emission Spectra Energy Levels and Spectral Charts-1.pdf ... Thus, emission spectra are experimental proof that electrons exist in definite, distinctive energy levels in an atom. Questions: 1. How can the difference in the brightness of spectral lines be explained? 2. According to the modern theory of the atom, where may an atom's electrons be found? 3. How do electrons become "excited"? 4.
PDF Emission Spectra and Energy Levels energy produced by the movement of an electron from one discrete energy level to another. Thus, emission spectra are experimental proof that electrons exist at definite, distinctive energy levels in an atom. Problem: In this experiment, you will study the emission spectra of three elements: hydrogen, neon, and helium. You will
Emission spectra and energy levels worksheet pdf - United ... Worksheet 1.3 Emission spectra and electron configurations Each wavelength can be mathematically related to a definite quantity of energy produced by the movement of an electron from one discrete energy level to another. Thus, emission spectra are experimental proof that electrons exist in definite, distinctive energy levels in an atom.
Lesson Worksheet:Emission and Absorption Spectra | Nagwa In this worksheet, we will practice determining the composition of a material from the features that appear in the spectrum of light coming from it. Q1: A scientist has a sample of an unknown gas. In order to identify it, she shines a continuous spectrum of white light through the gas and observes which wavelengths of light are absorbed by it.
Sch4u answers - bodycoach-online.de Include the day/time and place your electronic signature. doc The Arrhenius Equation was proposed by Svante Arrhenius in 1889 as a simple, yet accurate representation of the number of successful reactions as a function of temperature: k = A*exp (-E A / (RT)) Where E A is the activation energy ( J ), R is the universal gas constant ( J mol-1 K-1 ...
Lesson Worksheet:Atomic Emission Spectroscopy | Nagwa In this worksheet, we will practice explaining the emission of fixed colors of light by metal atoms and using line spectra in identifying elements. Q1: The atomic emission spectra of sodium and thallium are shown below. When placed into the flame of a Bunsen burner, sodium gives a vivid yellow flame.
Emission Spectra Worksheets & Teaching Resources | TpT This activity worksheet, reviews the emission spectrum of hydrogen including the electromagnetic spectrum, where students identify frequency, energy and wavelength. Labeling and describing absorption spectra and emission spectra, including a continuous spectrum, to identify the differences between t Subjects: Chemistry, Physical Science, Physics
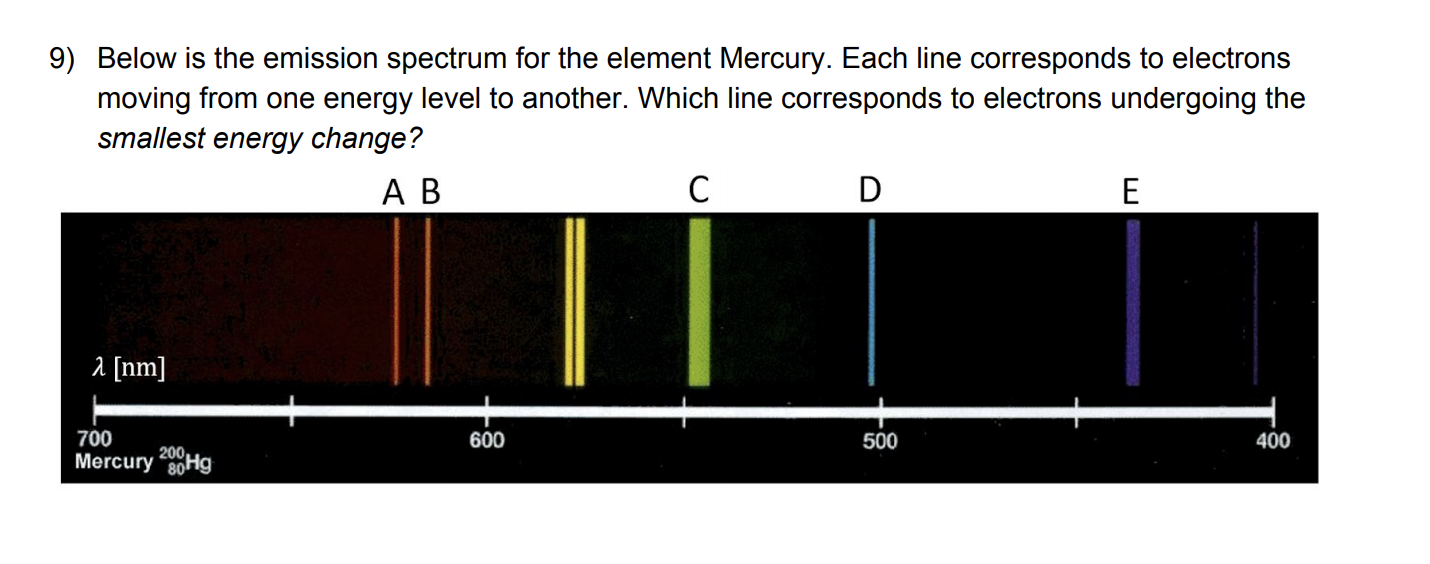
Quiz & Worksheet - Line Emission Spectra | Study.com Worksheet 1. Which of the following best describes the reason that line emission spectra contain lines? The energy levels in an atom are discrete and only certain emission wavelengths are possible....
EmissionSpectraEnergyLevelsPractice.pdf - Name Date Pd ... movement of an electron from one discrete energy level to another. Thus, emission spectra are experimental proof that electrons exist in definite, distinctive energy levels in an atom. Questions: 1. How can the difference in the brightness of spectral lines be explained? The thickness (brightness) depends on the number of photons.
Bohr’s Theory of the Hydrogen Atom | Physics Figure 1. Niels Bohr, Danish physicist, used the planetary model of the atom to explain the atomic spectrum and size of the hydrogen atom. His many contributions to the development of atomic physics and quantum mechanics, his personal influence on many students and colleagues, and his personal integrity, especially in the face of Nazi oppression, earned him a prominent place …
Chemistry Quizzes - Study.com Interested in seeing how well you know a particular chemistry concept? Take Study.com's brief multiple-choice quiz. Obtain rapid feedback and results to …
Emission Spectra and Energy Levels Worksheet - Studyres Thus, emission spectra are experimental proof that electrons exist in definite, distinctive energy levels in an atom. Questions: 1. How can the difference in the brightness of spectral lines be explained? 2. According to the modern theory of the atom, where may an atom's electrons be found? 3. How do electrons become "excited"? 4.
Spectra and energy levels | Teaching Resources A series of discussions and demonstrations looking at spectra and emission levels. Tes classic free licence. Report this resource to let us know if it violates our terms and conditions. Our customer service team will review your report and will be in touch. £0.00.
PDF Emission Spectra - Michigan State University Emission Spectra Data Collection and Analysis Do the following steps for each light source (5 in total) after you've set it up: 1. Observe the light source through the diffraction grating. You may need to hold the grating at an angle to observe the interference pattern.







0 Response to "45 emission spectra and energy levels worksheet"
Post a Comment