44 ionic and metallic bonding chapter 7 worksheet answers
Chapter 7 Ionic And Metallic Bonding Test Answers ... 17 terms Chapter 7 ionic and metallic bonding test answer key. Name three properties of metals that can be explained by metallic bonding. The attraction of valence electrons for positive metal ions. Free floating valence electrons are attracted to positively charged metal cations. The attraction of valence electrons for positive metal ions. PDF Section 7.1 ionic and metallic bonding answers Chapter 7 Ionic and Metallic Bonding 7.3 Bonding in Metals 7.1 Ions Date: 2020-2-20 | Size: 21.4Mb 7 Metallic Bonds and Metallic Properties [Answer to question 4] Metal bonds are the forces of attraction between free-floating valence electrons and positively charged metal ions.
Ionic and Metallic Bonding- Chapter 7 Flashcards | Quizlet metallic bond the attraction of valence electrons for positive metal ions. number of valence electrons in Group 6A 6 the charge on the resulting ion when an aluminum atom loses its valence electrons 3+ Metals are good conductors of electricity they contain mobile valence elctrons ionic compound
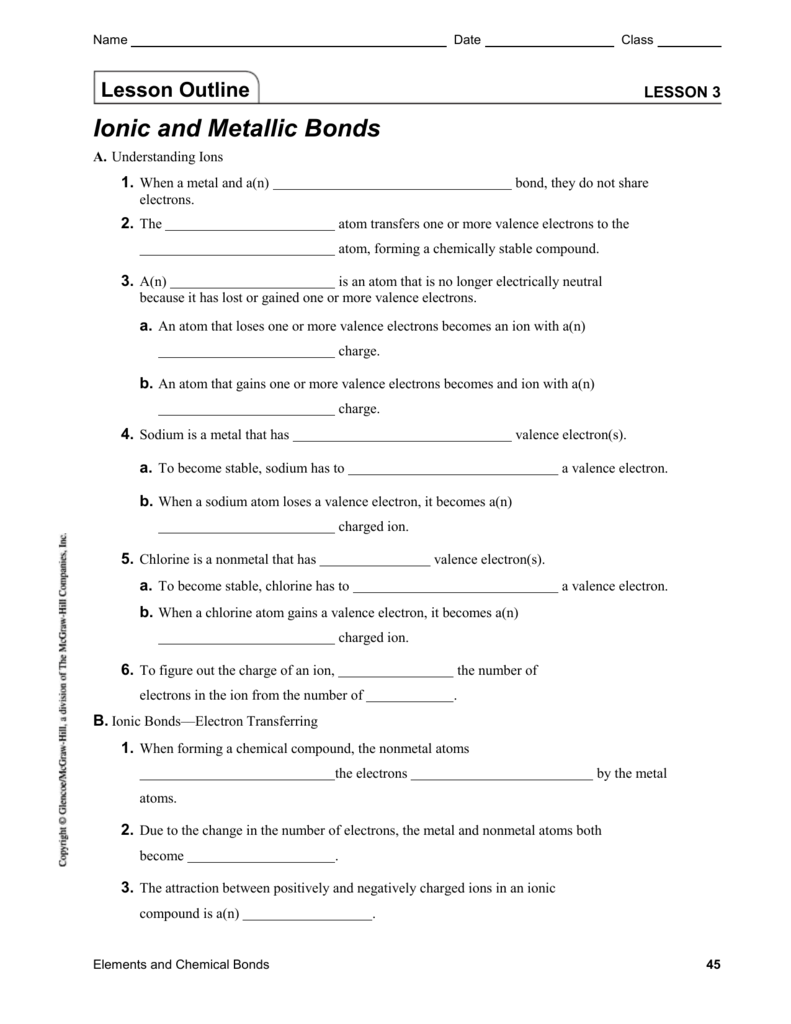
Ionic and metallic bonding chapter 7 worksheet answers
Chemistry Chapter 7 Test; Ionic and Metallic Bonding ... a negative ion formed when a halogen atom gains an electron Metallic Bond the force of attraction that holds metals together; it consists of the attraction of free-floating valence electrons for positively charged metal ions. Alloy a mixture composed of two or more elements, at least one of which is a metal
Ionic and metallic bonding chapter 7 worksheet answers. Chemistry Chapter 7 Test; Ionic and Metallic Bonding ... a negative ion formed when a halogen atom gains an electron Metallic Bond the force of attraction that holds metals together; it consists of the attraction of free-floating valence electrons for positively charged metal ions. Alloy a mixture composed of two or more elements, at least one of which is a metal




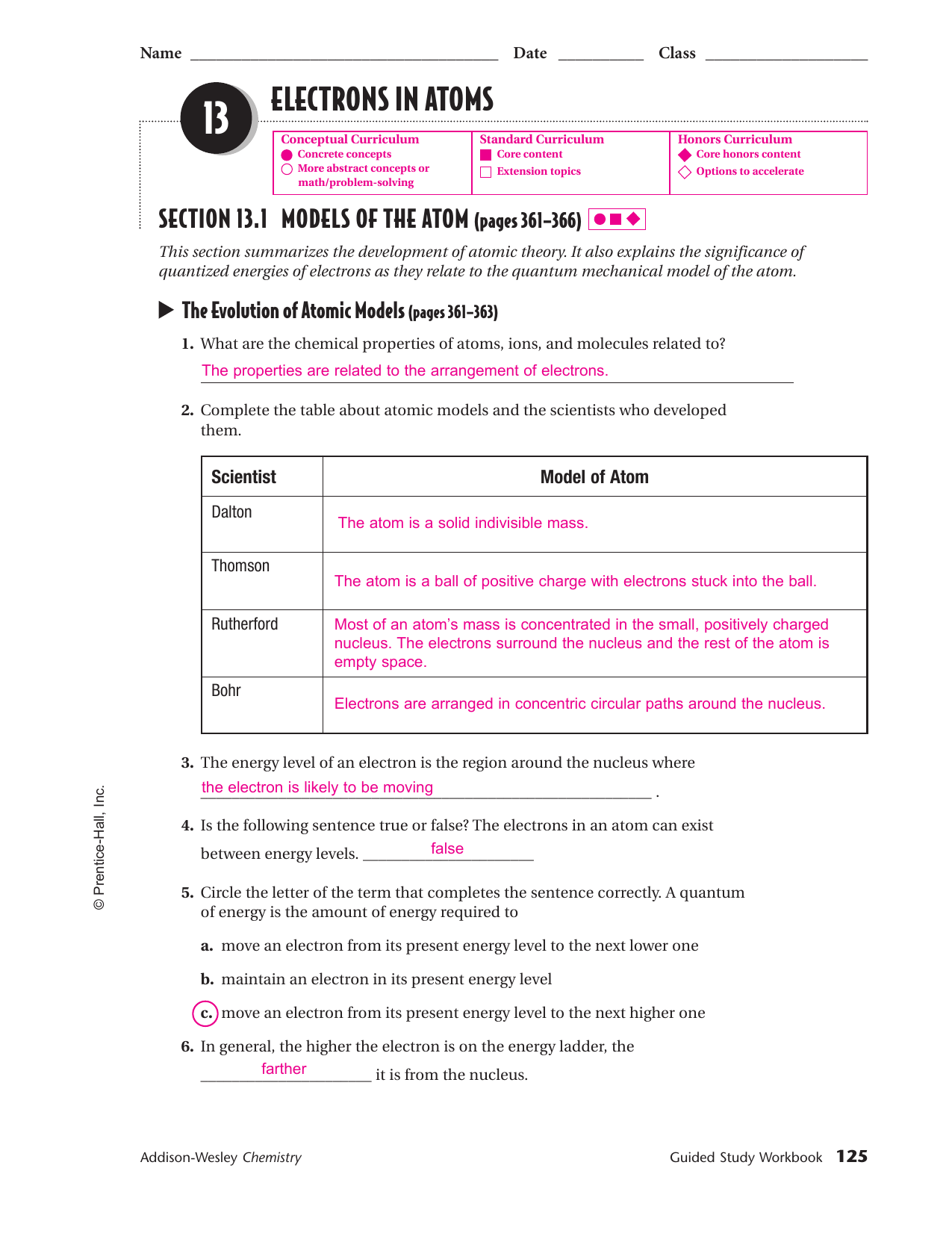
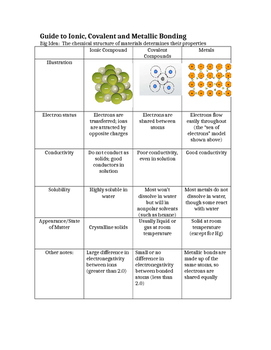
0 Response to "44 ionic and metallic bonding chapter 7 worksheet answers"
Post a Comment