45 specific heat worksheet answers
Calculating Specific Heat Worksheet Answers - safss.msu.edu Specific Heat Worksheet Name (in ink): C = q/mAT, where q = heat energy, m = mass, and T = temperature Remember, AT = (Tfinal ̶ Tinitial). Show all work and proper units. Answers are provided at the end of the worksheet without units. 1. A 15.75-g piece of iron sorbs 1086.75 joules of heat energy, and its temperature changes from 25 0 1750C. PDF Specific Heat and Heat Capacity Worksheet - New Providence School District Specific Heat and Heat Capacity Worksheet DIRECTIONS: Use q = (m)(Cp))(ΔT) to solve the following problems. Show all work and units. Ex: How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°C to 55°C, if the specific heat of aluminum is 0.90 J/g°C? 1.
PDF Specific Heat Capacity Problems Worksheet Answers - Women in Technology ... Before discussing Calculating Specific Heat Worksheet Answers, you need to recognize that Knowledge can be your answer to a better the next day, along with studying doesn't just stop the moment the school bell rings.Of which getting claimed, many of us provide you with a a number of basic yet helpful posts along with design templates made ...

Specific heat worksheet answers
PDF Isd 622 Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 6.75x104 joules of heat, and its temperature changes from 320C to 57 oc cp. û5Dc 37500CP 100.0 of 40C water is heated until its temperature is 370C. If the specific heat of water is 4.18 J/ábc, calculate the amount of heat needed to cause this rise in temperature. PDF Specific heat and calorimetry (heat lost=heat gained) worksheet answer key Specific heat and calorimetry (heat lost=heat gained) worksheet answer key Go to Mixing Two Amounts of Water Worksheet #2 Back to Thermochemistry Menu Problem #1: Determine the final temperature when 10.0 g of steam at 100.0 °C mixes with 500.0 grams of water at 25.0 °C. Solution: This problem is like 9 and 10 in Worksheet #2 with one difference. Freezing and Boiling Points - CliffsNotes where K f is a constant that depends on the specific solvent and m is the molality of the molecules or ions solute. Table 1 gives data for several common solvents. Table 1 gives data for several common solvents.
Specific heat worksheet answers. Heat Capacity Calorimetry Worksheet answers - StuDocu What is the specific heat of the metal? The energy absorbed by the water is q= m x cp x ∆T q= 75 x 4 J/g * C x (28 - 24) q = 1 x 10 3 J Therefore the energy lost by the object is -1 x 10 3 J So we can solve for the heat capacity of the object cp = q / m x ∆T cp = -1 x 10 3 J / 26 x (82 oC - 28) cp = 0/g * C PDF Problem solving specific heat and latent heat worksheet answers Problem solving specific heat and latent heat worksheet answers (We consider isolated systems, unless otherwise stated, so that no heat is lost to the environment and the principle of conservation of energy can be applied to work out the answer.) 1) How much heat is needed to transform 500g of ice at -20 oC into water at 50 oC ? PDF Specific Heat Capacity Worksheet - HYDESCIENCE 2. How is knowing the specific heat of an object useful? The table to the right list the specific heat (Heat Capacity) of four common materials Aluminum, Beryllium, Gold, and Graphite. Use this table to answer questions 3 - 7 3. If we were to heat up each of the objects to a temperature of 250oC. And we were adding the same amount of heat per ... Honors Chemistry Worksheet - Specific Heat - Quia Use dimensional analysis whenever possible. (ANSWERS) 1. A 500 g piece of iron changes 7°C when heat is added. How much heat energy produced this change in temperature? (Ans. 2,000 J) 2. When 300. cal of energy is lost from a 125 g object, the temperature decreases from 45.0°C to 40.0°C. ... Honors Chemistry Worksheet - Specific Heat ...
Search Printable 5th Grade Science Worksheets - Education Browse Printable 5th Grade Science Worksheets. Award winning educational materials designed to help kids succeed. Start for free now! PDF Isd 622 The initial temperature of the glass is 20.00C Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 6.75x104 joules of heat, and its temperature changes from 320C to 570C. 100.0 mL of 40C water is heated until its temperature is 370C. PDF Worksheet- Calculations involving Specific Heat Worksheet- Calculations involving Specific Heat 1. For q= m c Δ T : identify each variables by name & the units associated with it. q = amount of heat (J) m = mass (grams) c = specific heat (J/g°C) ΔT = change in temperature (°C) 2. Heat is not the same as temperature, yet they are related. Explain how they differ from each other. Specific Heat And Calorimetry Worksheet Answer Key - Google Groups To calorimetry worksheet answers worked out in a liquid, specific heat is lower heat is negative and metal type of vaporization. Use calorimetry worksheet answer keys each worksheet answers in...
Specific Heat Worksheets With Answers Specific Heat formula is made use of to find the specific heat of any given material, its mass, heat gained or temperature difference if some of the variables are given.This value for Cp is actually quite large. This (1 cal/g.deg) is the specific heat of the water as a liquid or specific heat capacity of liquid water. PDF Specific Heat Wksht20130116145212867 - Chandler Unified School District 1. A 15.75-g piece of iron sorbs 1086.75 joules of heat energy, and its temperature changes from 25 0 1750C. Calculate the specific heat capacity of iron. = 'C ' Q 5) 2. How many joules of heat are neeaea Fo ratse the temperature of 10.0 g of aluminum from 220C to 550C, if the specific heat of aluminum is 0.90 J/g0C? 3. Calculation Tools | Greenhouse Gas Protocol Country-specific tools: Customized for particular developing countries. Sector-specific tools: Principally designed for the specific sector or industry listed, though they may be applicable to other situations. Tools for countries and cities: These tools help countries and cities track progress toward their climate goals. PDF Specific Heat Practice Worksheet - Houston Independent School District Specific Heat Practice Worksheet 1. An aluminum skillet weighing 1.58 kg is heated on a stove to 173 oC. Suppose the skillet is cooled to room temperature, ... A 1.0 kg sample of metal with a specific heat of 0.50 KJ/KgC is heated to 100.0C and then placed in a 50.0 g sample of water at 20.0C. What is the final temperature of the metal and
What Is Energy? - Lesson - TeachEngineering Sep 14, 2005 · thermal energy: Heat energy produced when the molecules of a substance vibrate. The more heat a substance has, the more rapid the vibration of its molecules. Heat energy flows from places of higher temperature to places of lower temperature. Assessment Pre-Lesson Assessment. Discussion: Ask students the following questions: What is energy?
PDF sch4u-specific heat and heat capacity worksheet with answers itself absorbs a negligible amount of heat, calculate the amount of heat absorbed for the reaction. (ans. 2.79 kJ heat absorbed) 11. Titanium metal is used as a structural material in many high-tech applications such as jet engines. What is the specific heat of titanium in J/g°C if it takes 89.7 J to raise the temperature of a 33.0g block by 5 ...
DOC Specific Heat Worksheet - Socorro Independent School District 1. A 15.75-g piece of iron absorbs 1086.75 joules of heat energy, and its temperature changes from 25°C to 175°C. Calculate the specific heat capacity of iron. 2. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°C to 55°C, if the specific heat of aluminum is 0.90 J/g°C? 3.
PDF Latent heat and Specific heat capacity questions. Latent heat and Specific heat capacity questions. 1. How much water at 50°C is needed to just melt 2.2 kg of ice at 0°C? 2. How much water at 32°C is needed to just melt 1.5 kg of ice at -10°C? 3. How much steam at 100° is needed to just melt 5 kg of ice at -15°C? 4. A copper cup holds some cold water at 4°C.
PDF Extra Practice - Calculating Specific Heat Worksheet Answers Created Date: 7/15/2013 12:50:20 PM
specific-heat-worksheet-answers.pdf - | Course Hero specific-heat-worksheet-answers.pdf - specific-heat-worksheet-answers.pdf - School Gov. Thomas Johnson High; Course Title MONEY, FINANCE & ECON 101; Uploaded By DoctorFogFinch3. Pages 4 Ratings 100% (1) 1 out of 1 people found this document helpful; This preview shows page 1 - 4 out of 4 pages.
PDF Specific heat capacity No. Question Answer 5 100.0 g of water is cooled from 30.10 qC to 25.05qC. How much heat energy is released? 6 100.0 g of water at 25.00qC absorbs 100 J of heat. What is its final temperature? 7 A stone weighing 2.0 g absorbs 5.0 J of heat and warms by 3.0qC. What is the specific heat capacity of the stone? 8 80.0 g of propanone was heated from
Specific Heat Capacity - Worksheet (Key) | PDF | Thermodynamic ... - Scribd Specific Heat Capacity - Worksheet (Key) - Free download as PDF File (.pdf), Text File (.txt) or read online for free. Specific Heat Capacity - Worksheet (Key) ... Ch 9 Answers. Ibrahim A Said. Impulse and Momentum. Stone Wang. Friction. Ibrahim A Said. HPX2x12. dzuka. JN1387 - Swivel Support Daily Meeting 210318.
PDF Calculating Specific Heat 50 g of gold with a specific heat of 0.129 is heated to 115 0C; the gold cools until the final ... q = m x c x ΔT q metal = _____ J Problems: Write the Formula, set up problem, and answer in correct units. 1. Ethanol which has a specific heat of 2.44 (J/gx0C). The temperature of 34.4 g of ethanol increases from 25 0C to 78.8 0C ...
Heat, Temperature & Thermal Energy Worksheets [MCQ] (Physics) A material with greater specific heat a. warms up more quickly. b. requires less energy to get hot. c. always has a higher temperature. d. none of the above; Answers to MCQ Worksheet (set 1) Multiple Choice. d; b; b; d; d; d; d; Transfer of Thermal Energy - MCQ Worksheet (with answer) - set 2. A pot resting on a hot stovetop heats up ...
PDF Worksheet- Introduction to Specific Heat Capacities Water has the highest specific heat capacity and metal has the lowest. 6. Here are the heat capacities of the four substances: 0.10 cal/g °c, 0.25 cal/g °c, 1.0 cal/g °c, & 0.2 cal/g °c. Match & then labeleach substance with its specific heat capacity on the graph. See graph above. 7.
Class 6 Science Chapter 2 Components of Food Worksheet The worksheet test paper for 6 Science Components of Food is very helpful for students who wish to get good marks in exams. We have also provided answers to the worksheet questions in this article. Class 6 Science Components of Food Worksheets with Answers Chapter 2 – PDF. 1. Fill in the Blanks: (i) _____ and _____ mainly provide energy to ...
DOC Specific Heat Worksheet - Winston-Salem/Forsyth County Schools (Cp of H2O = 4.184 J/g °C) 11. How much energy is required to heat 120.0 g of water from 2.0 °C to 24.0 °C? (Cp of H2O = 4.184 J/g °C) 12. How much heat (in J) is given out when 85.0 g of lead cools from 200.0 °C to 10.0 °C? (Cp of Pb = 0.129 J/g °C) 13.
Specific Heat Worksheet (1).pdf - Specific Heat Worksheet... - Course Hero Use the table to answer questions 5-9Q Work Answer with Units! 1 Find the amount of heat (Q) needed to raise the temperature of 5.00 g of a substance from 20.0qC to 30.0qC if the specific heat of the substance is 2.01 J/gqC.100.5 J 2 A metal with a specific heat of 0.780 J/gqC requires 45.0 J of heat to raise the temperature by 2.00qC.
Problems on Specific Heat with Answers for AP Physics Specific Heat Problems. Problem (1): An chunk of steel with a mass of 1.57 kg absorbs net thermal energy of 2.5\times 10^ {5} 2.5×105 J and rises its temperature by 355°C. What is the specific heat of the steel? Solution: the specific heat of a substance is defined as the energy transferred Q Q to a system divided by the mass m m of the ...
Class 9 Science CBSE worksheet for matter in our Surroundings 5.The amount of heat energy required to convert 1 kg of solid into liquid at atmospheric pressure at its melting point 6. The amount of heat energy required to convert 1 kg of liquid at its boling point to vapour or gas without any change in temperature 7. Steam 8. By increasing pressure on liquid 9. Air,earth ,fire and water 11. Liquid other ...











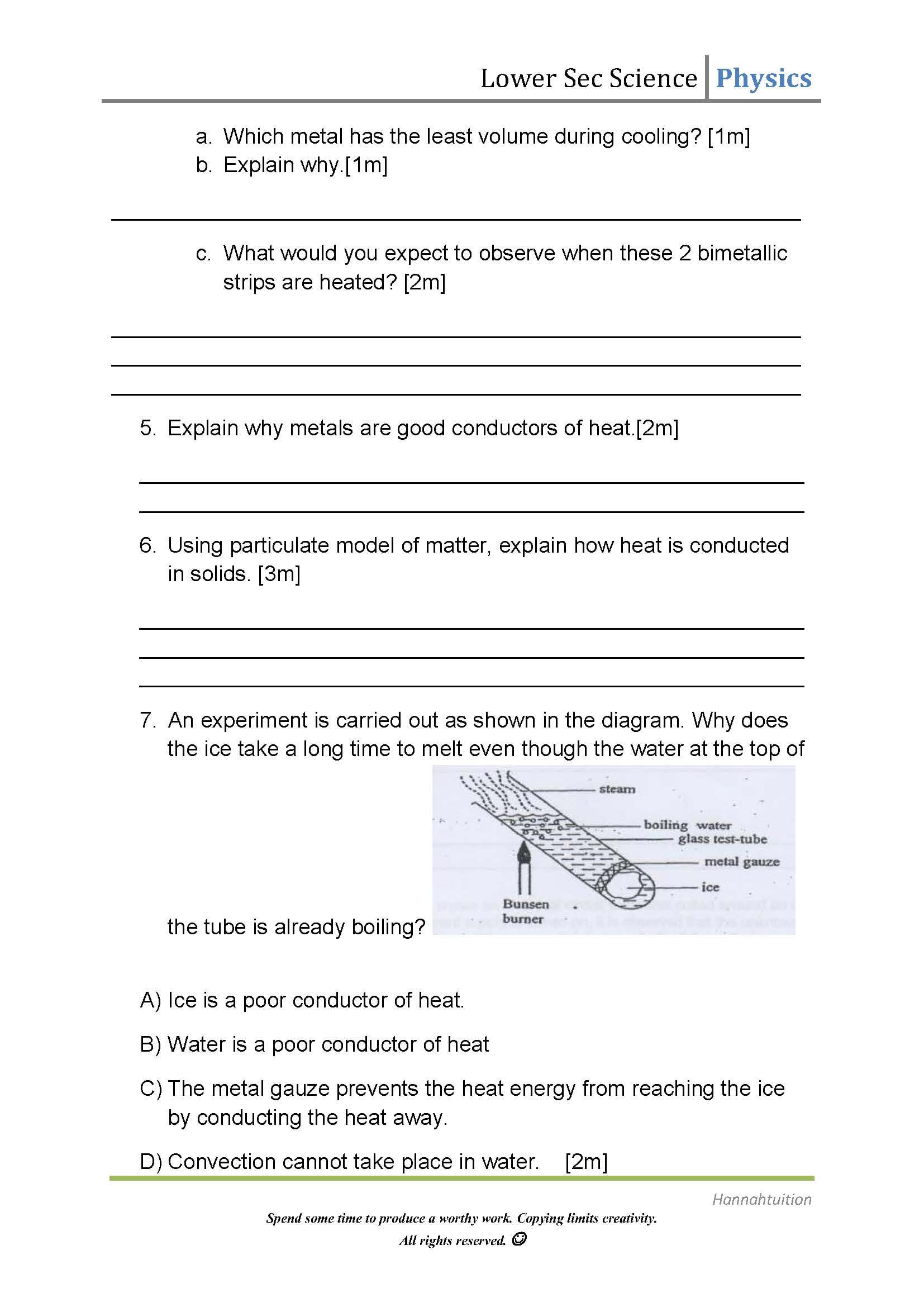

0 Response to "45 specific heat worksheet answers"
Post a Comment