38 atomic theory review worksheet
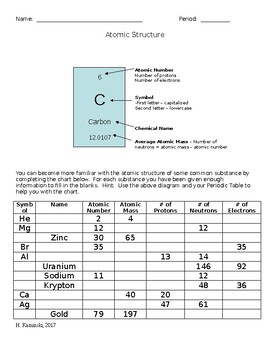
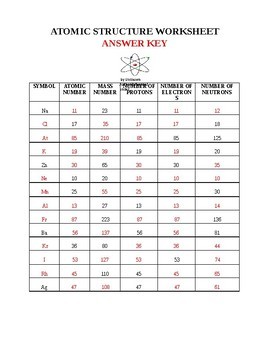
Mr. Christopherson / Atomic Structure - McLean County Unit District No. 5 Worksheets *Vocabulary: Atomic Structure pdf *Atomic Number and Mass Number pdf *Ions and Subatomic Particles pdf *Development of the Atomic Theory pdf (history of the Atom paragraph) *Light Problems pdf *Half-life of Radioactive Isotopes pdf Quantum Mechanics and Electron Configuration (paragraph) *Atom, Mass, and the Mole pdf Atomic Structure Test Review Worksheet Answers Atomic Structure Review Worksheet Key 1. 12 found below symbol on periodic table 2. Na atomic number 11 is Sodium 3. 4 mass - protons 7-3 = 4 4. 10 protons = electrons 5. 5 found by looking for the atomic number ... True 0 atomic mass units for an electron 1 atomic mass unit for protons and neutrons 15. True The electron cloud is mainly empty ...
Atomic Theory Review Worksheet Atomic Theory Review Worksheet - Worksheets are obviously the spine to students getting to know and grasping ideas taught through the teacher. Making your personal worksheets is easy, and it allows you to include just the right fabric that you want to make certain your scholars can gain knowledge of and decide to memory. Here are instructions ...
Atomic theory review worksheet
PDF Atomic Theory - Mr. Krohn 8th grade science - Home SECTION 1 DEVELOPMENT OF THE ATOMIC THEORY, 1. An atom is the smallest particle of an element that keeps its properties. 2. in a regular or repeating pattern, 3. when new information is found that does not fit the original theory, 4. positive, 5. negative, 6. a beam of small, positively charged particles, Worksheet Development Of Atomic Theory True Or False Answers ATI Dosage Calculations Final Exam 1 - Version 1 KEY Name - StuDocu. Medication order: 35 mg of a medication by mouth tidPatient weight: 99 pounds Safe dose range: 2 to 4 mg/kg/day This is a safe medication order. A) True B) False Answer _____ A) True. A child weighs 65 pounds. Convert to kilograms. Lesson 9 Atomic Theory Review Worksheet (1) (1).docx Use the following information to determine the atomic mass of carbon. Two isotopes are known: carbon-12 (mass = 12.000 amu) and carbon-13 (mass = 13.003 amu). Their relative abundance's are 98.9% and 1.10% respectively. ( 12. 000 amu x 0.989 ) + ( 13.003 amu x 0.11 ) = 13.30 amu, 14.
Atomic theory review worksheet. Atomic Structure Review Worksheet.doc - Course Hero Atomic Review Worksheet Section 1. Early Atomic Theory Indicate whether each statement below is TRUE or FALSE1. Ancient philosophers regularly performed controlled experiments. 2. Philosophers formulated explanations about the nature of matter based on their own experiences. 3. Both Democritus and Dalton suggested that matter is made up of atoms. Atomic Theory Review Worksheet - Naturalism View lesson 9 atomic theory review worksheet (1) (1).docx from phys 1310 at university of windsor. Neatly provide complete, detailed, yet concise responses to the following questions and problems. The diameter of the atom is about 100,000 . Atomic model of matter worksheet and key 5. Relate the interaction potential to the forces between molecules. DOC Chemistry Webquest #1: Introduction to Atoms Worksheet Name the date and inventor of the modern version of the Atomic Theory He developed the plum pudding model and also was the first to discover the electron In 1909 this scientist demonstrated that the atom is mostly empty space with a small positively charged nucleus containing most of the mass and low mass negatively charged particles orbiting ... Atomic Structure Review Sheet Answers - myilibrary.org Atomic Structure Review Worksheet Key 1. 12 found below symbol on periodic table 2. Na atomic number 11 is Sodium 3. 4 mass - protons 7-3 = 4 4. 10 protons = electrons 5. 5 found by looking for the atomic number ... True 0 atomic mass units for an electron 1 atomic mass unit for protons and neutrons 15.
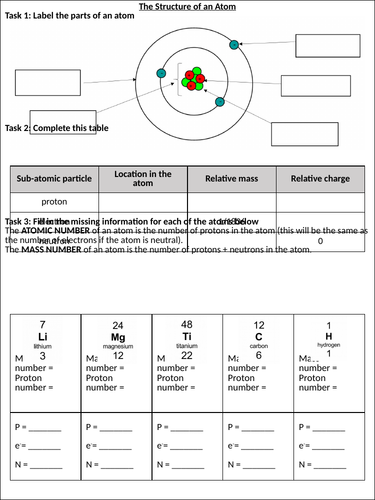
Unit 5: Atomic Theory - Science Day 1: Review of Science 10 and Atomic Model Project PDF Atomic Theory - Review Sheet - Concord Consortium Atomic Theory - Review Sheet, You should understand the contribution of each scientist. I don't expect you to memorize dates, but I do want to know what each scientist contributed to the theory. Democretus - 400 B.C. theory that matter is made of atomsBoyle 1622 -1691, defined elementLavoisier 1743-1797, Atomic Theory and Structure Review worksheet ID: 2835540 Language: English School subject: Chemistry Grade/level: 11 Age: 16-18 Main content: Atomic Theory and Structure Other contents: Unit Test Review Add to my workbooks (0) Download file pdf Embed in my website or blog Add to Google Classroom DOC Chapter 4 Review Worksheet - Currituck County Schools English chemist and schoolteacher who formulated a theory to describe the structure and chemical reactivity of matter in terms of atoms, 1. Who did this experiment? 2. Draw in what happened? 3. What properties did he find for the pieces? 4. How did he describe the atom? 5. Who did this experiment? 6. Describe the alpha particles, 7.
Atomic Theory Review Worksheet Answers - qstion.co It is composed of protons neutrons and electrons. Balancing nuclear equations worksheets answer key. Atomic theory review worksheet answers. Relate the interaction potential to the forces between molecules. Which two subatomic particles are located in the nucleus of an atom. Quiz & practice tests with answer key pdf, electronic devices ... DOC History of the Atom Worksheet - sfponline.org 1. What did J.J. Thompson discover? 2. What is the charge of an electron? 3. What are cathode rays made of? 4. Why do electrons move from the negative end of the tube to the positive end? 5. What was Thompson working with when he discovered the cathode rays? Lord Ernest Rutherford (1871 - 1937): Atomic Theory Review Worksheets & Teaching Resources | TpT Atomic Theory Review -- Protons, Neutrons, Electrons, by, Chemistry Wiz, 2, $3.00, PDF, Compatible with, Teach your students about the atom and subatomic particles using this detailed worksheet! Perfect for any chemistry or physical science classroom. DOCX Saint Louis Public Schools / Homepage Chemistry Unit 3 Atoms and the Periodic Table Name: _____ Atomic History Worksheet . Complete the following . Summary . table with the contribution of each scientist and the Atomic Structure the. y were using: Logical bits of matter, Solid Spheres, Plum Pudding, Nuclear. Model, Bohr Model . or . Quantum Mechanics
Atomic Theory Worksheets Teaching Resources | Teachers Pay Teachers Great 10 problem worksheet covering Atomic Theory & History Chemistry I standards to use as homework, additional practice, tutoring, substitute plans, and more! Solutions included!Print or virtual use through Easel.Included: 1. Rank in Historical Order- Atomic Theories2. True/False- Structure of Atom ( 5 questions)3.
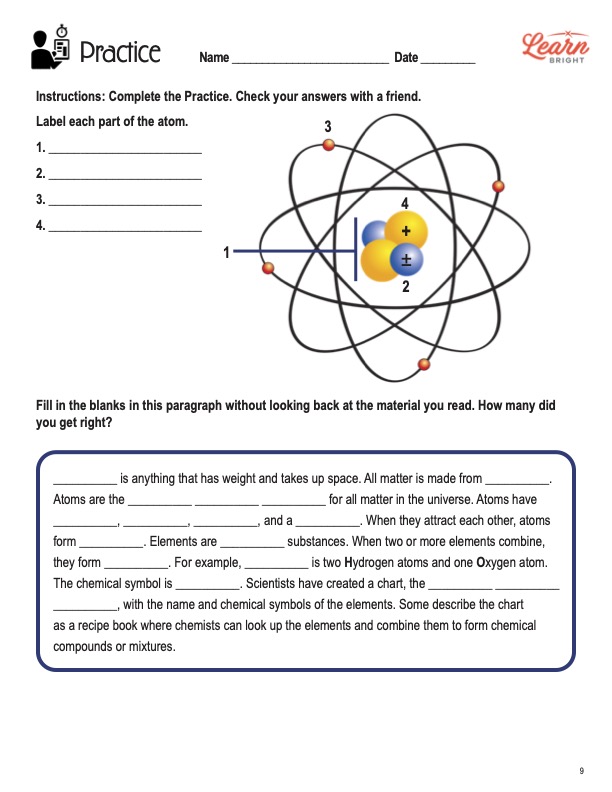
Atomic Structure Review Worksheet Teaching Resources | TpT This lesson will teach students about the parts of an atom; nucleus, protons, neutrons, electrons, and their charges. Students will also learn about atomic theory and how the structure of an atom changed over time. Finally, students will learn how to count atoms to find atomic numbers and mass. Like this atomic structure activity?
Lesson 9 Atomic Theory Review Worksheet (1) (1).docx Use the following information to determine the atomic mass of carbon. Two isotopes are known: carbon-12 (mass = 12.000 amu) and carbon-13 (mass = 13.003 amu). Their relative abundance's are 98.9% and 1.10% respectively. ( 12. 000 amu x 0.989 ) + ( 13.003 amu x 0.11 ) = 13.30 amu, 14.
Worksheet Development Of Atomic Theory True Or False Answers ATI Dosage Calculations Final Exam 1 - Version 1 KEY Name - StuDocu. Medication order: 35 mg of a medication by mouth tidPatient weight: 99 pounds Safe dose range: 2 to 4 mg/kg/day This is a safe medication order. A) True B) False Answer _____ A) True. A child weighs 65 pounds. Convert to kilograms.
PDF Atomic Theory - Mr. Krohn 8th grade science - Home SECTION 1 DEVELOPMENT OF THE ATOMIC THEORY, 1. An atom is the smallest particle of an element that keeps its properties. 2. in a regular or repeating pattern, 3. when new information is found that does not fit the original theory, 4. positive, 5. negative, 6. a beam of small, positively charged particles,



















0 Response to "38 atomic theory review worksheet"
Post a Comment