41 calculating average atomic mass worksheet answers
2.3: Calculating Atomic Masses (Problems) - Chemistry LibreTexts (a) atomic number 9, mass number 18, charge of 1− (b) atomic number 43, mass number 99, charge of 7+ (c) atomic number 53, atomic mass number 131, charge of 1− (d) atomic number 81, atomic mass number 201, charge of 1+ (e) Name the elements in parts (a), (b), (c), and (d) Answer a. p: 9; n: 9; e: 10. Answer b. p: 43; n: 56; e: 36. Answer c. p: 53; n: 78; e: 54. Answer d. p: 81; n: 120; e: 80. Answer e PDF AVG Atomic Mass WS Key - Livingston Public Schools Subject: Image Created Date: 9/28/2012 8:47:13 AM
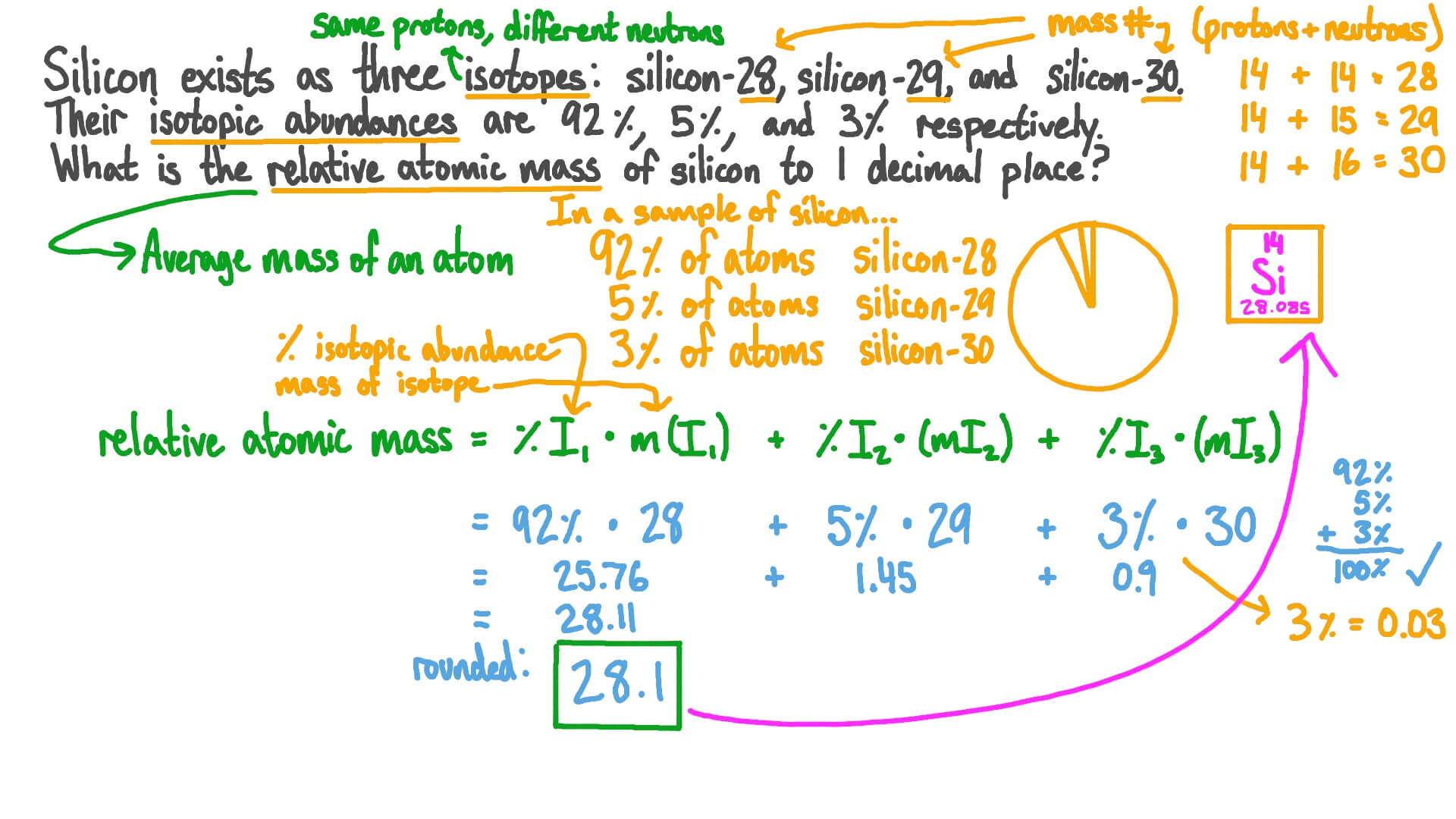
PDF Calculating Average Atomic Mass Worksheet - SI PROGRAM Calculating Average Atomic Mass Worksheet: 1) Three isotopes of Silicon occur in nature: Atomic Mass: 27.97693 amu 28.97649 amu 29.97377 amu Isoto es of Silicon: Silicon-28 Silicon-29 Silicon-30 Percent Abundance: 92.23% 4.68% 3.09% Calculate the average atomic mass for the three isotopes of Silicon. (-9 Y(.o ( zq.q 7377) 0/0

Calculating average atomic mass worksheet answers
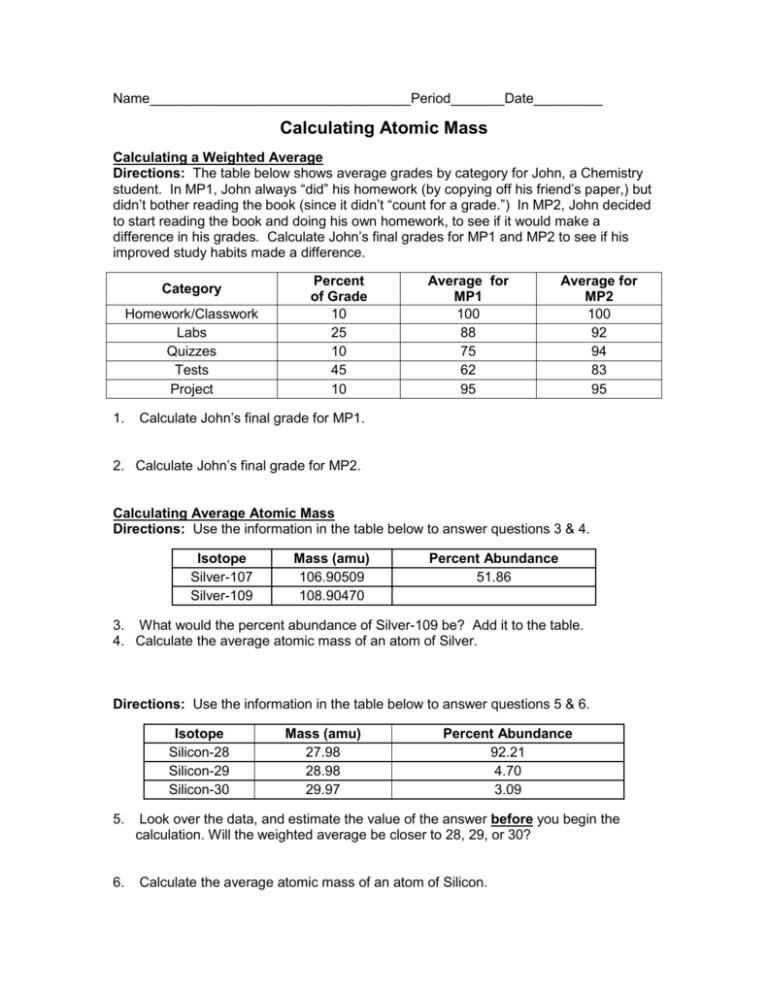
DOC Calculating Average Atomic Mass Worksheet Name______________________ 2. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance and atomic masses are: 69.2% for mass of 62.93u . 30.8% fora mass of 64.93u. Calculate the average atomic mass of copper. 3. Calculate the average atomic mass of sulfur. if 95.00% of all sulfur atoms have a mass of 31.972 u, 0.76% PDF KMBT 654-20131024112244 - Berger's Chemistry Class The average atomic mass is the weighted average of all the isotopes of an element. Example: A sample of cesium is 75% 133Cs, 20% 132Cs and 5% 134Cs. What is its average atomic mass? Answer: .75 x 133 = 99.75 .20 x 132 = 26.4 .05 x 134 = Total = 132.85 amu = average atomic mass Determine the average atomic mass of the following mixtures of isotopes. Calculating Average Atomic Mass - Study.com Calculating Average Atomic Mass High School Chemistry Skills Practice 1. Carbon has three isotopes, namely Carbon-12, Carbon-13, and Carbon-14. C-12 has a mass of 12.000 amu and is 98.89%...
Calculating average atomic mass worksheet answers. atomic number worksheet - Microsoft ATOMS LESSON PLAN - A COMPLETE SCIENCE LESSON USING THE 5E METHOD OF. 8 Pics about ATOMS LESSON PLAN - A COMPLETE SCIENCE LESSON USING THE 5E METHOD OF : Atomic Structure Worksheet 8th Grade Answer Key - Thekidsworksheet, Calculating Average Atomic Mass - YouTube and also Calculating Average Atomic Mass - YouTube. Worked Chemistry Problem Examples - ThoughtCo Nov 22, 2019 · Included in this list are printable pdf chemistry worksheets so you can practice problems and then check your answers. You may also browse chemistry problems according to the type of problem. You may also browse chemistry problems according to the type of problem. PDF Isotope Practice Worksheet - Chemistry Answer: The atomic mass of boron is 10.811; therefore, boron-11 is more abundant because the mass number is closer to the atomic mass. 8. Lithium-6 is 4% abundant and lithium-7 is 96% abundant. What is the average mass of lithium? Answer: 6.96 amu 9. Iodine is 80% 127I, 17% 126I, and 3% 128I. Calculate the average atomic mass of iodine. Answer: 126.86 amu DOC Chemistry Worksheet - Forestville Central High School Calculate the average atomic mass of gold with the 50% being gold-197 and 50% being gold-198. Calculate the average atomic mass of lithium, which occurs as two isotopes that have the following atomic masses and abundances in nature: 6.017 u, 7.30% and 7.018 u, 92.70%. Hydrogen is 99% 1H, 0.8% 2H, and 0.2% 3H. Calculate its average atomic mass.
Chemistry: Average Atomic Mass Worksheet Flashcards | Quizlet Calculate the average atomic mass for each element based on the natural abundance of its isotopes. Terms in this set (4) Find the average atomic mass for Li if 7.5% of Li atoms are 6Li with a mass of 6.0151223 amu and 92.5% are 7Li with a mass of 7.0160041 amu. (7.5 x 6.01251223) + (92.5 x 7.0160041) / 100; 45.0938417 + 648.9803793 / 100 = Calculating Average Atomic Mass Worksheet.pdf - Course Hero Calculating Average Atomic Mass Worksheet Highlight the black box to check your answer. 1. The term "average atomic mass" is a average, and so is calculated differently from a "normal" average. 2. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance are 69.2% and 30.8% respectively. 6.2 Solids, liquids and gases | Particle model of matter ... The mass of an object or a substance tells us how much matter it consists of. The greater the mass of an object, the more matter it contains. Mass is measured in kilograms (kg). When we measure the mass of small objects or small amounts of matter we often measure in grams (g) or even milligrams (mg). One kilogram is the same as 1000 grams. Lesson Worksheet:Percentage Isotopic Abundance | Nagwa In this worksheet, we will practice calculating percentage isotopic abundances from the relative atomic mass and isotopic masses. Q1: Chlorine has two stable isotopes, 3 5 C l and 3 7 C l , with atomic masses 34.9689 u and 36.9659 u respectively. The relative abundance of 3 7 C l in an average sample of chlorine is 3 7 3 5 C l C l = 0. 3 1 9 6.
Physics Quizzes | Study.com The Theory of Conservation of Mass-Energy . ... Average Speed & Velocity: Quiz & Worksheet for Kids . View Quiz. ... Calculating Displacement with Velocity & Time . Chemistry Quizzes | Study.com Calculating Molar Mass Using PV=nRT . ... Isotopes and Average Atomic Mass . View Quiz. Predict the Formation, Charge, and Formulas of Ions ... Comparing Mass in Solutions: Quiz & Worksheet for ... Calculating Average Atomic Mass Worksheet Answers (2022) - dev ... calculating-average-atomic-mass-worksheet-answers 1/1 Downloaded from dev.endhomelessness.org on September 8, 2022 by guest ... Getting the books calculating average atomic mass worksheet answers now is not type of challenging means. You could not solitary going next book accrual or library or borrowing from your associates to retrieve them. Atomic Mass Atomic Number Worksheets - K12 Workbook Displaying all worksheets related to - Atomic Mass Atomic Number. Worksheets are Chemistry work atomic number and mass number, Atomic structure review work answers, Mayfield high school, Chemistry atomic number and mass number, Atomic structure periodic table work answers, Chapter 4 chemical patterns work science quest, Atomic structure chapter 4 work answers, Honors chem atomic.
Worksheets | Telecom Fiji Worksheet 7 - Finding Rules – Finding the Values of Pronumerals ; Worksheet 8 - Metric Conversions(mm/cm/m/km) Worksheet 9 - Calculating Areas/Calculating Perimeters; Worksheet 10 - Rounding Off Numbers to the Nearest Centimeter/Meter/Kilometer
Calculating Average Atomic Mass Worksheet - Name ___Lisa ... - StuDocu Calculate the average atomic mass of Sulfur if: 95% of all Sulfur atoms have a mass of 31 u, 0% has a mass of 32 and 4% have a mass of 33. Show ALL work for full credit . 0 x 31 = 30. 0 x 32 = 0. 0 x 33 = 1. 7777777777775 = 32 u
PDF isotopic abundance practice problems - CHEMISTRY name: !suggested answers date: _____ ! isotopic abundance - practice problems The atomic mass for each element appearing on the periodic table represents the weighted average of masses for each individual isotope of an element. For example, the atomic mass of carbon is reported as 12.011 amu (atomic mass units). Carbon is composed primarily of two isotopes; carbon-12 and carbon-14.
PDF Average Atomic Mass Practice Problems - scott.kyschools.us 2. Calculate the average atomic mass of lithium, which occurs as two isotopes that have the following atomic masses and abundances in nature: 6.017 u, 7.30% and 7.018 u, 92.70%. 3. 2Hydrogen is 99% 1H, 0.8% H, and 0.2% 3H. Calculate its average atomic mass. 4. Calculate the average atomic mass of magnesium using the following data for three
pycse - Python3 Computations in Science and Engineering Python is a basic calculator out of the box. Here we consider the most basic mathematical operations: addition, subtraction, multiplication, division and exponenetiation. we use the func:print to get the output.
PDF CHM 4, PAL - atomic mass Student name - California State University ... to calculate a weighted average of its isotope masses. a. Use the equation in question 1 to calculate the atomic mass of an element that has two isotopes, each with 50.00% abundance. One isotope has a mass of 63.00 amu and the other has a mass of 68.00 amu. b. Recalculate the atomic mass if instead there is 80.00% of the 63.00 amu isotope and 20.00% of the 68.00 amu isotope.
PDF Practice Problems - Denton ISD 37. Calculate the average atomic mass of chlorine. 2. Copper has two isotopes. Copper-63, which has an atomic mass of 62.93 u and copper-65, which has an atomic mass of 64.93 u. In any sample of copper atoms, 69.1% will be copper-63 and 30.9% will be copper-65. Calculate the average atomic mass of naturally occurring copper. 3. One atom has 20 ...
Calculating Average Atomic Mass Worksheet - Name ... - StuDocu The term "average atomic mass" is a _____average, and so is calculated differently, from a "normal" average. Explain how this type of average is calculated.-----The element Copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance and atomic masses are: 69% for mass of 62 30% for mass of 64. Calculate the average atomic mass of Copper.
PDF Henry County Schools / Overview Created Date: 9/18/2013 8:46:55 AM
PDF NAME Average Atomic Mass Worksheet: show all work. - Weebly The average atomic mass between these two isotopes is 63.546 amu. Calculate the actual atomic mass of 65Cu. 65Cu = 64.9278 amu 7) Magnesium consists of three naturally occurring isotopes. The percent abundance of these isotopes is as follows: 24Mg (78.70%), 25Mg (10.13%), and 26Mg (11.7%). The average atomic mass of the three isotopes is 24.3050 amu.
PDF KM 654e-20150908091438 - ms. adrangi's teaching site The relative abundance and atomic masses are 69.2% for a mass of 62.93amu and 30.8% for a mass of 64.93amu. Calculate the average atomic mass of copper. 93) (93.5 2. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 3 1.972 amu, 0.76% has a mass of 32.971 amu and 4.22% have a mass of 33.967amu.
Calculating Average Atomic mass Worksheet-1 ( 1).docx View Calculating Average Atomic mass Worksheet-1 ( 1).docx from ECON 123 at Sumner High. Calculating Average Atomic Mass (2C) Worksheet: 1. Three isotopes of Silicon occur in nature: Isotopes of. ... Build_an_Atom_Remote_Lab.docx; filename answer.docx. Francis Lewis High School.
Basic Atomic Structure Worksheet Key - Neshaminy School District have the atomic number. The mQSS of an element is the average mass of an element's naturally occurring atom, or isotopes, taking into account the Of each isotope. The q of an element is the total number of protons and neutrons in the of atom. The mass number is used to calculate the number of O in one atom of an element. In
Average Atomic Mass Practice Problems Quiz - Quizizz answer choices 40.00 amu 41.00 amu 35.00 amu impossible to tell Question 3 900 seconds Q. Calculate the average atomic mass of an element with the follow isotope information: 4.35% have a mass of 49.9461 amu, 83.79% have amass of 51.9405 amu, 9.50% have a mass of 52.9407 amu, and 2.36% have a mass of 53.9389 amu. answer choices 51.99 amu 52.19 amu
PDF Answers Key for Unit Worksheets - Livingston Public Schools 5) Naturally occurring chlorine that is put in pools is 75.53 percent 35Cl (mass 34.969 amu) and 24.47 percent 37Cl (mass = 36.966 amu). Calculate the average atomic mass. 6) Copper used in electric wires comes in two flavors (isotopes): Cu and "Cu. 63 Cu has an atomic mass of 62.9298 amu and an abundance of 69.09%.
Average Atomic Mass 2013 Worksheets - K12 Workbook *Click on Open button to open and print to worksheet. 1. Average Atomic Mass Problems key 2013 - 2. Chemistry: Average Atomic Mass Worksheet 3. Mass Worksheet: show all work. Name 4. 5. UNIT 3 6. Calculating Average Atomic Mass Worksheet Answers 7. Average Atomic Mass Answers - 8. Abundance of Isotopes Name Chem Worksheet 4-3












0 Response to "41 calculating average atomic mass worksheet answers"
Post a Comment