45 calculating average atomic mass worksheet
Atomic Mass Worksheets Teaching Resources | Teachers Pay Teachers This worksheet walks students through the steps of calculating the average atomic mass of an element. Students will then practice through a couple of word problems. This worksheet includes an explanation of what average atomic mass is, an example of how to calculate amu, one conceptual question, and six practice problems. KEY INCLUDED. Atomic Average Mass Teaching Resources | Teachers Pay Teachers This worksheet walks students through the steps of calculating the average atomic mass of an element. Students will then practice through a couple of word problems. This worksheet includes an explanation of what average atomic mass is, an example of how to calculate amu, one conceptual question, and six practice problems.
Worked Chemistry Problem Examples - ThoughtCo Nov 22, 2019 · Atomic Mass from Atomic Abundance; Atomic Weight Calculation; Average of a Set of Numbers; ... Calculating Molarity — Worksheet;

Calculating average atomic mass worksheet
calculating average atomic mass worksheet (1).doc - Name_... Calculate the average atomic mass of copper. 2. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.76% have a mass of 32.971amu and 4.22% have a mass of 33.967amu. 3. Calculate the average atomic mass of bromine. PDF Calculating Average Atomic Mass Worksheet Name - Solano Community College Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.76% has a mass of 32.971amu and 4.22% have a mass of 33.967amu. 4. Calculate the average atomic mass of bromine. One isotope of bromine has an atomic mass of 78.92amu and a relative abundance of 50.69%. Chemistry: Average Atomic Mass Worksheet Flashcards | Quizlet 10.81 = B Find the average atomic mass for Cl is 75.78% of Cl atoms are 35Cl with a mass of 34.96885271 amu and 24.22% are 37Cl with a mass of 36.96590260 amu. (75.78 x 34.96885271) + (24.22 x 36.965902060) / 100; 2649.939658 + 895.314161 / 100 = 35.45 = Cl
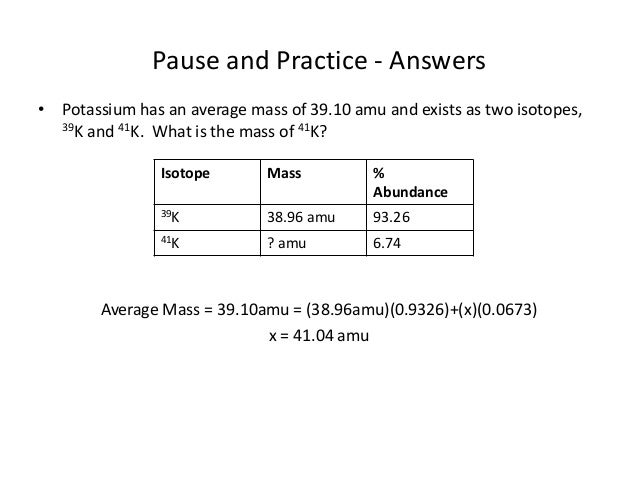
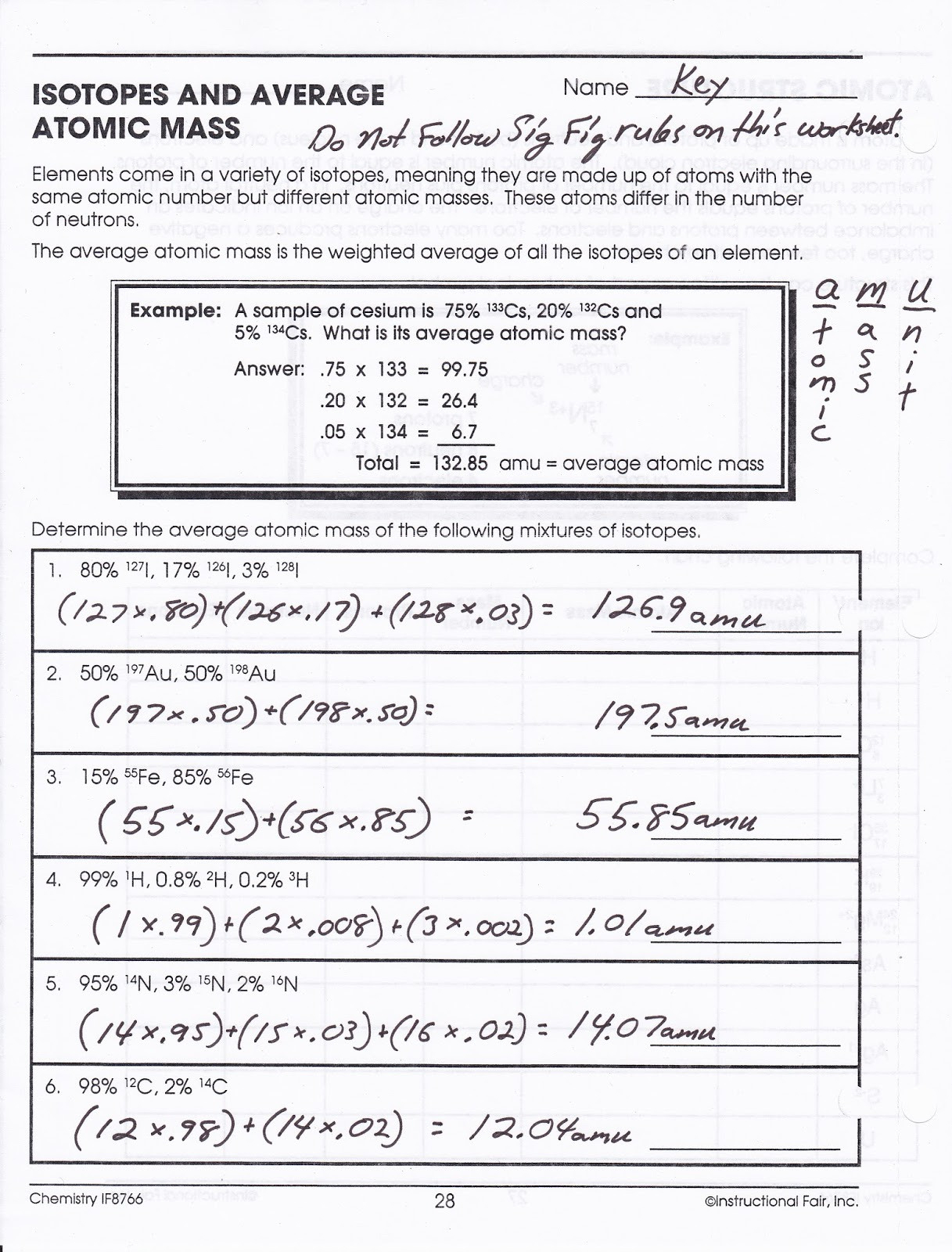
Calculating average atomic mass worksheet. PDF Calculating Average Atomic Mass Worksheet Name - Onstudy Academy The relative abundance and atomic masses are 69.2% for a mass of 62.93amu and 30.8% for a mass of 64.93amu. Calculate the average atomic mass of copper. 3. Calculate the average atomic mass of Sulfur if 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.76% have a mass of 32.971amu and 4.22% have a mass of 33.967amu. 4. Adam_Es-Sabir_-_Copy_of_Calculating_Average_Atomic_Mass_Worksheet ... Calculate the average atomic mass of copper. 2. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.76% has a mass of 32.971 amu, and 4.22% have a mass of 33.967 amu. 31. 972 amu , 0.76 % has a mass of 32.971 amu , and 4.22 % have a mass of 33.967 amu . 3. Lesson Worksheet:Percentage Isotopic Abundance | Nagwa In this worksheet, we will practice calculating percentage isotopic abundances from the relative atomic mass and isotopic masses. Q1: Chlorine has two stable isotopes, 3 5 C l and 3 7 C l , with atomic masses 34.9689 u and 36.9659 u respectively. The relative abundance of 3 7 C l in an average sample of chlorine is 3 7 3 5 C l C l = 0. 3 1 9 6. DOCX Calculating Average Atomic Mass Worksheet Name______________________ What is the average atomic mass? % x mass# = weighted part Answer: 0.75 x 133 = 99.750.20 x 132 = 26.40.05 x 134 = 6.7___ Total = sum of weighted parts = 132.85 amu 1. There are three isotopes of silicon. They have mass numbers of 28, 29 and 30. The average atomic mass of silicon is 28.086amu.
Realistic Designs A-G - Atomic Rockets This is the struts and fittings required to hold the transport or resupply package in the RLV, and to safely eject it from the RLV's cargo bay or whatever. The ASE mass is estimated to be 15% of the item mass. Example: if the transport has a mass of 12,377 kg, the ASE will be an additional 1,857 kg of struts and fittings. DOC Calculating Average Atomic Mass Worksheet Name______________________ Calculate the average atomic mass of copper. 2. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 32, 0.76% has a mass of 33 and 4.22% have a mass of 34. 3. The four isotopes of lead are shown below, each with its percent by mass abundance and the composition of its nucleus. PDF Atoms and Isotopes Worksheet Atoms and Isotopes Worksheet Fill in the table with the correct information Isotope Isotope notation Atomic # Protons Electrons neutrons Oxygen-16 8 8 8 8 ... Calculate the average atomic mass of chlorine if its isotopes and % abundance are as follows. Show work. Mass of Isotope % abundance 36.96590 24.47 (.2447)(36.96590) = 9.04556 ... DOC Calculating Average Atomic Mass Worksheet Name - Courses Calculate the average atomic mass of copper. 2. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.76% has a mass of 32.971amu and 4.22% have a mass of 33.967amu. 3. The three isotopes of lead are shown below, each with its percent by mass abundance and the composition of its nucleus.
Mass Calculations Worksheets - K12 Workbook *Click on Open button to open and print to worksheet. 1. CHM 4, PAL atomic mass Student name 2. Example Exercise 9.1 Atomic Mass and Avogadros Number 3. Mole Calculations Worksheet 4. Mole Calculation Worksheet 5. Mass, Volume and Density Review Worksheet 6. Worksheet: Calculating Empirical & Molecular Formulas 7. PDF NAME Average Atomic Mass Worksheet: show all work. and 24.47 percent 37Cl (mass = 36.966 amu). Calculate the average atomic mass. 6) Copper used in electric wires comes in two flavors (isotopes): 63Cu and 65Cu. 63Cu has an atomic mass of 62.9298 amu and an abundance of 69.09%. The other isotope, 65Cu, has an abundance of 30.91%. The average atomic mass between these two isotopes is 63.546 amu. PDF isotopic abundance practice problems - CHEMISTRY name: !suggested answers date: _____ ! isotopic abundance - practice problems The atomic mass for each element appearing on the periodic table represents the weighted average of masses for each individual isotope of an element. For example, the atomic mass of carbon is reported as 12.011 amu (atomic mass units). Carbon is composed primarily of two isotopes; carbon-12 and carbon-14. PDF Calculating Average Atomic Mass Worksheet - SI PROGRAM Calculating Average Atomic Mass Worksheet: 1) Three isotopes of Silicon occur in nature: Atomic Mass: 27.97693 amu 28.97649 amu 29.97377 amu Isoto es of Silicon: Silicon-28 Silicon-29 Silicon-30 Percent Abundance: 92.23% 4.68% 3.09% Calculate the average atomic mass for the three isotopes of Silicon. (-9 Y(.o ( zq.q 7377) 0/0
DOC Calculating Average Atomic Mass Worksheet Name______________________ Calculate the average atomic mass of copper. 3. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 u, 0.76% has a mass of 32.971u and 4.22% have a mass of 33.967u. 4. Naturally occurring strontium consists of four isotopes, Sr-84, Sr-86, Sr-87 and Sr-88. Below is the data concerning strontium:
How to Find Average Atomic Mass: 8 Steps (with Pictures ... - wikiHow A molecule of water has the chemical formula H 2 O, so it contains two hydrogen (H) atoms and one oxygen (O) atom. Hydrogen has an average atomic mass of 1.00794 amu. Oxygen atoms have an average mass of 15.9994 amu. The average mass of a molecule of H 2 O equals (1.00794) (2) + 15.9994 = 18.01528 amu, equivalent to 18.01528 g/mol.
PDF CHM 4, PAL - atomic mass Student name - California State University ... CHM 4, PAL - atomic mass Student name: 1 This worksheet will provide you with the necessary background and practice to be a pro ... to calculate a weighted average of its isotope masses. a. Use the equation in question 1 to calculate the atomic mass of an element that has two isotopes, each with 50.00% abundance. One isotope has a mass of
Calculating Average Atomic Mass Worksheet - Name ... - StuDocu The element Copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance and atomic masses are: 69% for mass of 62 30% for mass of 64. Calculate the average atomic mass of Copper. Show ALL work for full credit.-----Calculate the average atomic mass of Sulfur if: 95% of all Sulfur atoms have a mass of 31 u, 0% ...
PDF Average Atomic Mass Practice Problems - scott.kyschools.us 1. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? 2. Calculate the average atomic mass of lithium, which occurs as two isotopes that have the following atomic masses and abundances in nature: 6.017 u, 7.30% and
Chemistry Simulations | CK-12 Foundation Discover a new way of learning Chemistry using Real World Simulations
DOC Chemistry Worksheet - Livingston Public Schools Calculate the average atomic masses. Use the atomic mass for significant digits. SHOW ALL WORK FOR CREDIT! What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177.0, 27 have a mass of 178.0, 14 have a mass of 179.0, and 35 have a mass of 180.0? 178.55amu; use three significant digits: 179amu
Worksheets | Telecom Fiji Worksheet 7 - Finding Rules – Finding the Values of Pronumerals ; Worksheet 8 - Metric Conversions(mm/cm/m/km) Worksheet 9 - Calculating Areas/Calculating Perimeters; Worksheet 10 - Rounding Off Numbers to the Nearest Centimeter/Meter/Kilometer
DOC Calculating Average Atomic Mass Worksheet Name______________________ Calculate the average atomic mass of copper. 3. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.76% has a mass of 32.971amu and 4.22% have a mass of 33.967amu. 4. The four isotopes of lead are shown below, each with its percent by mass abundance and the composition of its nucleus.
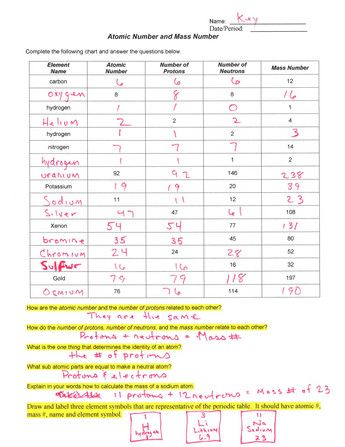
Basic Atomic Structure Worksheet Key - Neshaminy School District have the atomic number. The mQSS of an element is the average mass of an element's naturally occurring atom, or isotopes, taking into account the Of each isotope. The q of an element is the total number of protons and neutrons in the of atom. The mass number is used to calculate the number of O in one atom of an element. In
Chemistry Quizzes | Study.com Calculating Molar Mass Using PV=nRT . ... Isotopes and Average Atomic Mass . View Quiz. Predict the Formation, Charge, and Formulas of Ions ... Comparing Mass in Solutions: Quiz & Worksheet for ...
How to Calculate Average Atomic Mass | Chemistry | Study.com How to Calculate Average Atomic Mass Step 1: Identify the percentage of each isotope in the composition of the element and its mass. Step 2: For each isotope, multiply its mass by the percent. Step...
Physics Quizzes | Study.com The Theory of Conservation of Mass-Energy . ... Average Speed & Velocity: Quiz & Worksheet for Kids . View Quiz. ... Calculating Displacement with Velocity & Time .
Calculating Average Atomic Mass Worksheet - Name ___Lisa ... - StuDocu The relative abundance and atomic masses are: 69% for mass of 62 30% for mass of 64. Calculate the average atomic mass of Copper. Show ALL work for full credit. 69/100 x 62 = 43. 30/100 x 64 = 19. ggghvhvhvhvhhv= 63-----Calculate the average atomic mass of Sulfur if: 95% of all Sulfur atoms have a mass of 31 u, 0% has a mass of 32 and 4% have a ...








0 Response to "45 calculating average atomic mass worksheet"
Post a Comment