39 5.1 models of the atom worksheet answers
Section 5.1 Models Of The Atom Answers - acscu.net Chapter 5.1 Revising the Atomic Model Page 140 5.1 Lesson Check #1-7 1. The Bohr model propose that an electron is found only in specific circular paths or orbits around the nucleus. Electrons in Bohr's model have specific energies. These specific energies of an electron are called energy levels. 2. 5.1 Review - Name Date Class MODELS OF THE ATOM Section... - Course Hero A metal crystallizes with a face-centered cubic lattice. The edge of the unit cell is 408 pm. Calculate the number of atoms in the unit cell and diameter of the metal atom. ( For FCC , edge = r 8 ) Q&A Calculate the temperature of 2.50 g CO2gas occupies 5.60 liters at 789 torr inoC. Q&A Bookmarked 0 Recently viewed CHEMISTRY Chemistry 5.1 Review
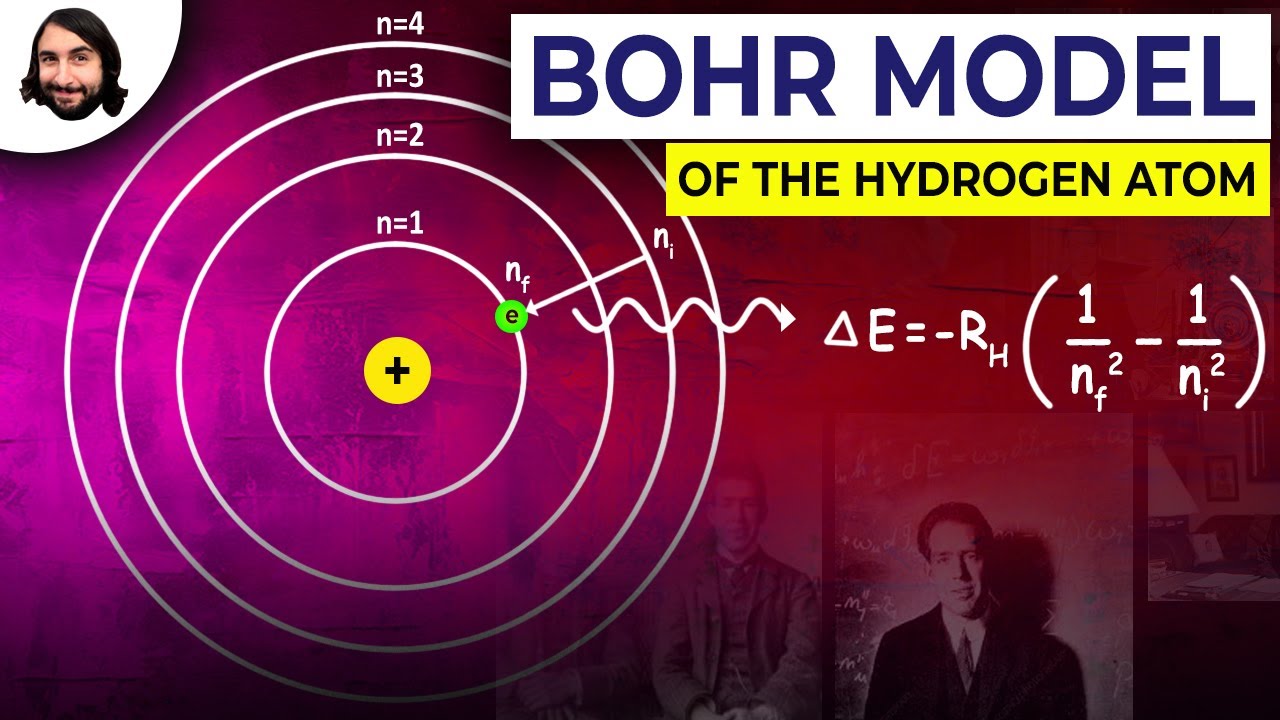
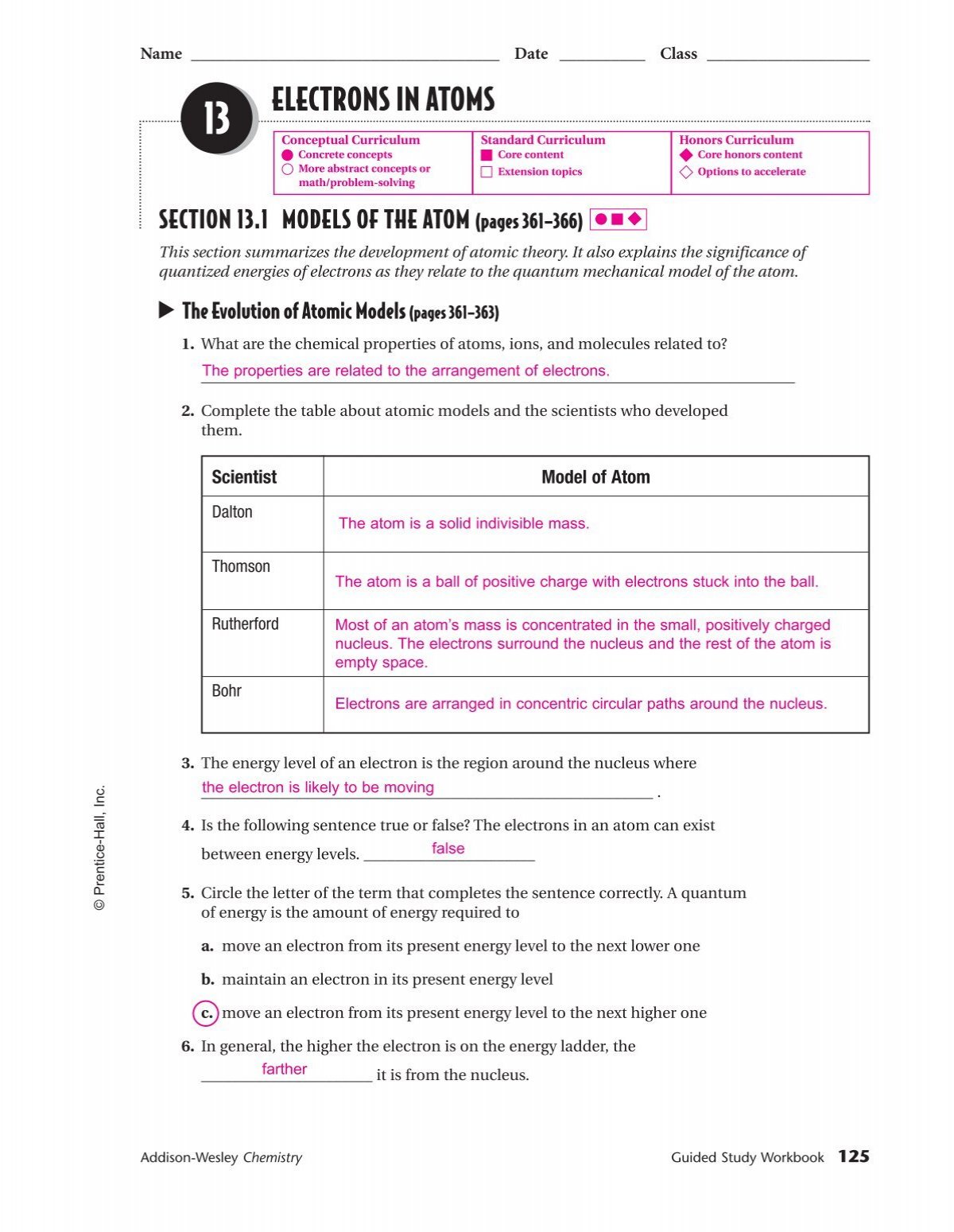
PDF SECTION 5.1 MODELS OF THE ATOM (pages 127-132) - Notre Dame High School tum mechanical model of the atom. The Development of Atomic Models (pages 127-128) 1. Complete the table about atomic models and the scientists who developed them. 2. Is the following sentence true or false? The electrons in an atom can exist between energy levels. _____ The Bohr Model (pages 128-129) 3. What is a small, discrete unit of ...
5.1 models of the atom worksheet answers
PDF History of atomic theory worksheet answer key Table of contents. 11 best images of atom worksheets with answer keys atoms. What type of charge does an electron have? Some of the worksheets displayed are 3 06 atomic structure wkst, atomic structure work, atomic structure, … Build an atom worksheet answer key. Weather station models worksheet | earth science lessons. It was from reliable on PPTX Chapter 5 - Electrons in Atoms - Henry County Schools Section 5.1 - Models of the Atom. The Rutherford's model of the atom did not explain how an atom can emit light or the chemical properties of an atom. Plum Pudding Model Rutherford's Model. The Bohr Model. Niels Bohr studied the hydrogen atom because it was the most simplistic. 13.1-Review-Answers.pdf - LPS Explain the significance of quantized energies of electrons as they relate to the quantum mechanical model of the atom. Key Terms energy level.
5.1 models of the atom worksheet answers. PDF Chapter 5 Atoms and Bonding - Chino Valley Unified School District The of an atom consists of a nucleus of protons and neutrons, surrounded by a cloud of moving electrons. ... answer the following questions. Chapter 5 Atoms and Bonding Comparing Molecular and Ionic Compounds ... model of solid metals explains their ability to conduct heat and electricity, the ease with PDF Chapter 5.1 Revising the Atomic Model - Mr.Nguyen's Pre AP Chemistry 5. In an atom, the electrons occupy certain fixed energy levels, to move from one energy level to another requires the emission or absorption of an exact of energy, or quantum. Thus the energy of an electron is said to be quantized. 6. a. 3 b. 1 c.3 d.5 7. The Bohr model limits electrons to specific circular paths. The quantum mechanical 5.1 models of the atom Flashcards | Quizlet Summarize the development of atomic theory ( short answer ) 1. Dalton proposed that matter was made of indestructible particles called Atoms 2. Thompson's proposed that the atomic model in which negatively charged electrons were embedded in a positively charged Mass. 3. Rutherford discovered that atoms are mainly empty space 4. Worksheet 5.1 - Models of the Atom.pdf - Course Hero _____ The Bohr Model of the atom says that: a. Electrons exist at different energy levels in orbit around the nucleus. b. Electrons can move between energy levels when they gain or lose energy. c. Electrons cannot exist in between an energy level. d. All of the above. 2. _____Which of the following best fits Thomson's model of the atom? a.
5.1 The Nuclear Model of the Atom - Save My Exams CIE IGCSE Physics Topic Questions. Home / IGCSE / Physics / CIE / Topic Questions / 5. Nuclear Physics / 5.1 The Nuclear Model of the Atom / Multiple Choice Questions 5.1 Flashcards | Quizlet The electron probability clouds for atomic orbitals are spherical in shape. ST The number of sub levels in an energy level is equal to the square of the principle quantum number of that energy level AT The maximum number of electrons that can occupy the fourth principle energy level of an atom is 32. AT Chapter 5.1 Models of the Atom Flashcards | Quizlet The higher an electron is on the energy ladder... the farther it is away from the nucleus What is a quantum? the amount of energy required to move an electron from one energy level to another Is the amount of energy an electron gains or loses always the same? no The higher the energy level occupied by an electron, the less... [FREE] 5.1 Models Of The Atom Quiz Answers | latest Answers to Review Questions for Atomic Theory Quiz #1 nucleus of the atom and that electrons are flying in a random electron cloud around the nucleus in Rutherford's model, the atom is mostly empty space and this is where the electrons are found 19. Compare and contrast the models of the atom that were proposed by Rutherford and Bohr.
Answer Key - Secton 5.1 Questions - Section 5.1 Assessment... For the electronic transition from n=2 to n=4 in the hydrogen atom. Calculate the energy Calculate the wavelength. Q&A. ... Answer Key - Mass Movement Section 5-3 and Chapter 5 Review Questions. notes. 1. Lecture Quiz 4 Solution. Indiana University, Bloomington. BIOL 111. Evolution; Chemistry (12th Edition) Chapter 5 - Electrons in Atoms - GradeSaver Chapter 5 - Electrons in Atoms - 5.1 Revising the Atomic Model - 5.1 Lesson Check - Page 132: 5 Answer Quantized energies means that electrons are moved between energy levels by gaining or losing a certain amount of energy. Work Step by Step 5.1 Models Of The Atom Answer Key Pdf - Wakelet 5.1 Models Of The Atom Answer Key Pdf - Wakelet. John Cox @JohnCox770. PDF 5.1 Models of the Atom The$Developmentof$Atomic$Models$ - Skyline Chemistry Models of the Atom > The$Bohr$Model$ - Electrons$are$found$only$in$specific$circular$paths,$ or$orbits,$around$the$nucleus.$ $ 5.1
modern atomic theory worksheet 12 Best Images Of Label An Atom Worksheet - Drawing Atoms Worksheet . worksheet periodic table label atom labeling worksheeto drawing atoms via. ... Section 5.1 Models Of The Atom Worksheet Answer Key - Ampirdesign ampirdesign.blogspot.com. Unit 2 - Atomic Theory - Mrs. Watson's Science Class mrswatsonscience.weebly.com. key.
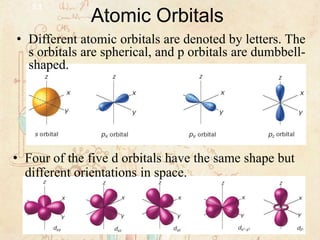
5.1 Models of the Atom Section Review Flashcards | Quizlet The electron probability clouds for atomic orbitals are spherical in shape Sometimes True (ST) The number of sublevels in an energy level is equal to the square of the principal quantum number of that energy level Never True (NT) The maximum number of electrons that can occupy the fourth principal energy level of an atom is 32 Always True (AT)
PDF 5.1 Models of the Atom - Weebly Models of the Atom > The Quantum Mechanical Model The propeller blade has the same probability of being anywhere in the blurry region, but you cannot tell its location at any instant. The electron cloud of an atom can be compared to a spinning airplane propeller. 5.1 . 11/18/14 8
5.1 Models Of The Atom Review Answers - acscu.net 51 models of the atom worksheet answers. 512 identify the new proposal in the bohr model of the atom. That quest continues in this chapter as scientists pursued an understanding of how electrons were arranged within atoms. Section 51 core teaching resources section 51 review transparencies t57 technology interactive textbook with chemasap ...
5.1 models of the atom Flashcards | Quizlet Start studying 5.1 models of the atom. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
️Models Of Atoms Worksheet Answers Free Download| Qstion.co Atomic structure worksheet pdf answers. Bohr atomic models worksheet answers. Models of atoms worksheet answers 5.1 models of the atom worksheet answers. Atoms isotopes and ions teacher answer key in this laboratory activity you will use chips and the information in a reference sheet to make models of atoms isotopes and ions of various elements.
5.1 Models Of The Atom Answers - acscu.net [GET] 5.1 Models Of The Atom Answers | HOT! Question 5 (1 point) In the Bohr model of the atom, for a short arrow that points in toward the center/nucleus (e.g. from n = 2 to n = 1), select all of the following statements that are true. The arrow pointing in means that the electron is losing energy.
Chapter 5.1 Models of the Atom - StudyLib Chapter 5 Electrons in Atoms 5.1 Models of the Atom Chemistry Today we are learning to:1. Realize there were problems with Rutherford's model of the atom 2.
PDF 5.1 Models of the Atom 5 Section 5.1 Models of the Atom 127 5.1 Models of the Atom Aeronautical engineers use wind tunnels and scale models to simulate and test the forces from the moving air on each proposed design. The scale model shown is a physical model. However, not all models are physical. In fact, several theoretical models of the atom
5.1 models of the atom worksheet answer - ecoland-crimea.ru Rutherford's model fails to explain why objects change color when heated. Chapter 5 Electrons In Atoms Workbook Answers Some answers are provided for you. (Hint: Remember that the maximum number of electrons in the first three shells is 2 5.1 models of the atom worksheet answer 8 5.1 models of the atom worksheet answer and 8.)
CK-12 Chemistry - Basic Answer Key Chapter 5: Electrons in Atoms 5.2 The Bohr and Quantum Mechanical Models of the Atom ... Answers 1. The Bohr model for hydrogen had the electron traveling around the nucleus. It explained the emission lines for hydrogen, but did not provide an explanation for the different emissions of larger atoms. 2. When most substances are heated to high enough temperatures, they give ...
Section 5.1 Models of the Atom Worksheet The Development of Atomic Models pages 127128 1. Up to 24 cash back Bohrs Model of the Atom pages 113116 1. The question that arises is In what way are the subatomic particles. 52 EARLIER MODELS OF ATOM In section 51 you have learnt that the atom is divisible and contains three smaller particles in it. Electromagnetic radiation includes many types.
Chapter 5 Review textbook KEY.pdf KEY. 5. Practice Problems. In your notebook, solve the following problems. ELECTRONS IN ATOMS. SECTION 5.1 MODELS OF THE ATOM.
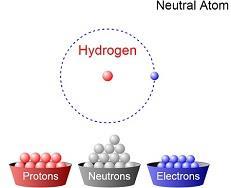
PDF 5 ATOMIC STRUCTURE - National Institute of Open Schooling On this basis he proposed a model for the structure of atom. According to his model, atoms can be considered as a large sphere of uniform positive charge with a number of small negatively charged electrons scattered throughout it, Fig. 5.4. This model was called as plum pudding model. The electrons represent the plums in the pudding made of ...
13.1-Review-Answers.pdf - LPS Explain the significance of quantized energies of electrons as they relate to the quantum mechanical model of the atom. Key Terms energy level.
PPTX Chapter 5 - Electrons in Atoms - Henry County Schools Section 5.1 - Models of the Atom. The Rutherford's model of the atom did not explain how an atom can emit light or the chemical properties of an atom. Plum Pudding Model Rutherford's Model. The Bohr Model. Niels Bohr studied the hydrogen atom because it was the most simplistic.
PDF History of atomic theory worksheet answer key Table of contents. 11 best images of atom worksheets with answer keys atoms. What type of charge does an electron have? Some of the worksheets displayed are 3 06 atomic structure wkst, atomic structure work, atomic structure, … Build an atom worksheet answer key. Weather station models worksheet | earth science lessons. It was from reliable on




























0 Response to "39 5.1 models of the atom worksheet answers"
Post a Comment