45 isotopes and average atomic mass worksheet
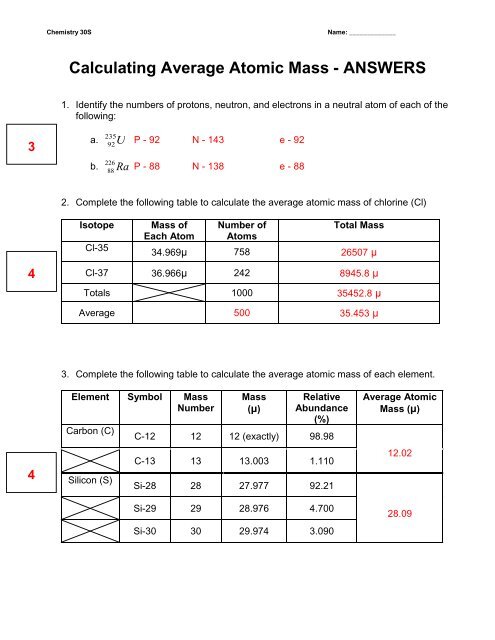
PDF isotopic abundance practice problems - CHEMISTRY name: !suggested answers date: _____ ! isotopic abundance - practice problems The atomic mass for each element appearing on the periodic table represents the weighted average of masses for each individual isotope of an element. For example, the atomic mass of carbon is reported as 12.011 amu (atomic mass units). Carbon is composed primarily of two isotopes; carbon-12 and carbon-14. PDF Atoms and Isotopes Worksheet Atoms and Isotopes Worksheet Fill in the table with the correct information Isotope Isotope notation Atomic # Protons Electrons neutrons Oxygen-16 8 8 8 8 ... Calculate the average atomic mass of chlorine if its isotopes and % abundance are as follows. Show work. Mass of Isotope % abundance 36.96590 24.47 (.2447)(36.96590) = 9.04556 ...
Isotopes And Average Atomic Mass Worksheet Answers not discover the broadcast Isotopes And Average Atomic Mass Worksheet Answers that you are looking for. It will very squander the time. However below, subsequent to you visit this web page, it will be thus categorically easy to acquire as capably as download guide Isotopes And Average Atomic Mass Worksheet Answers

Isotopes and average atomic mass worksheet
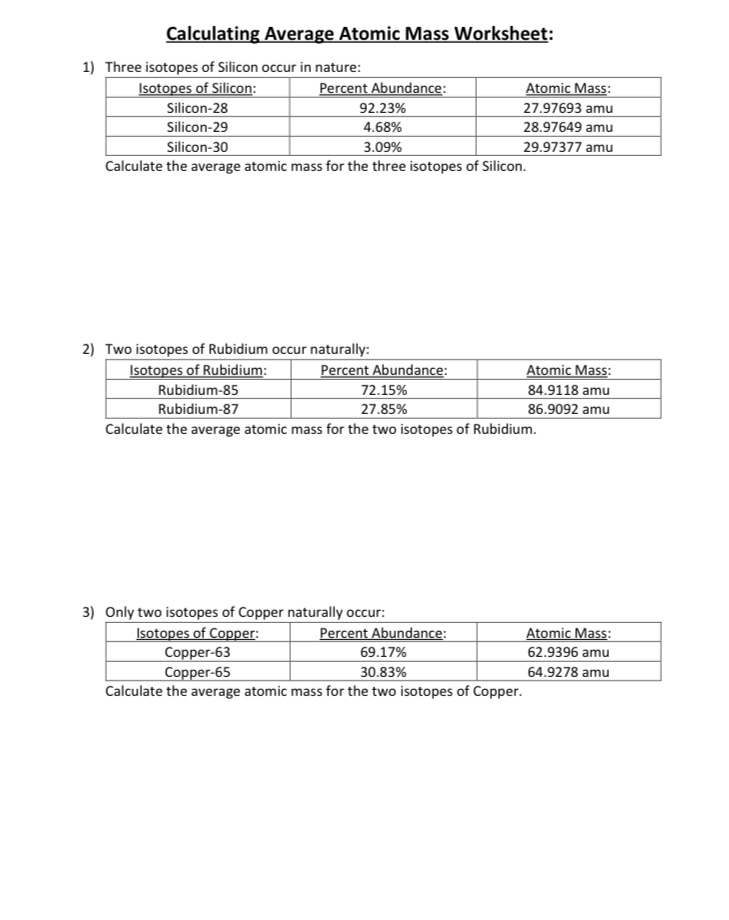
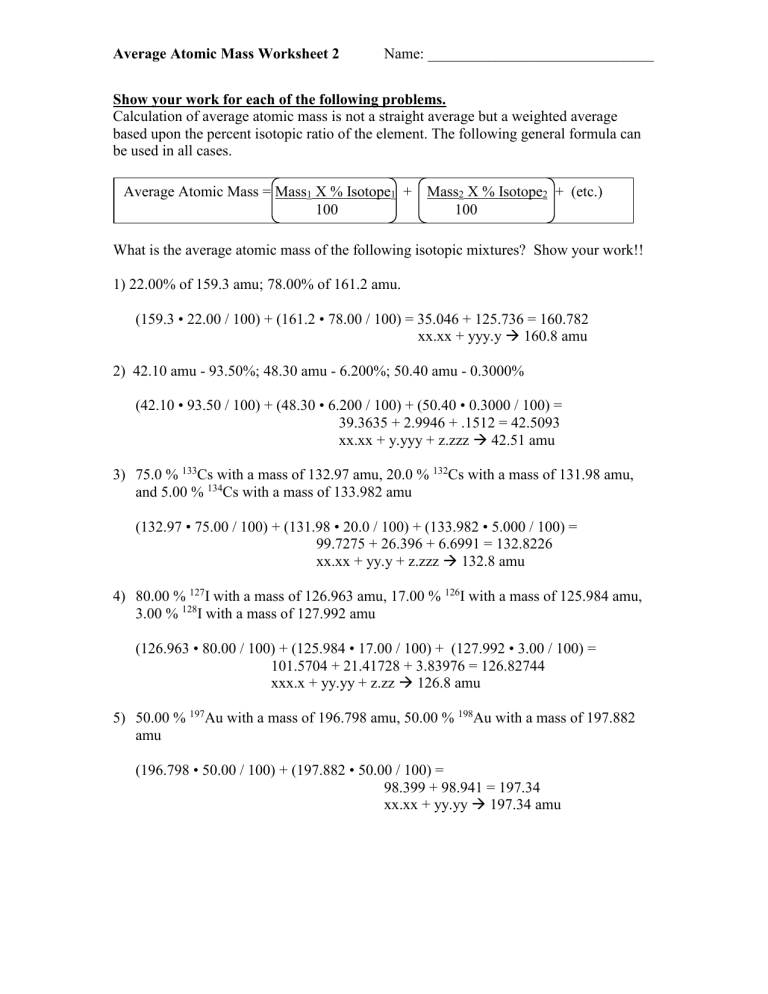
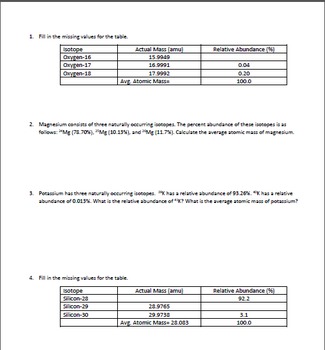
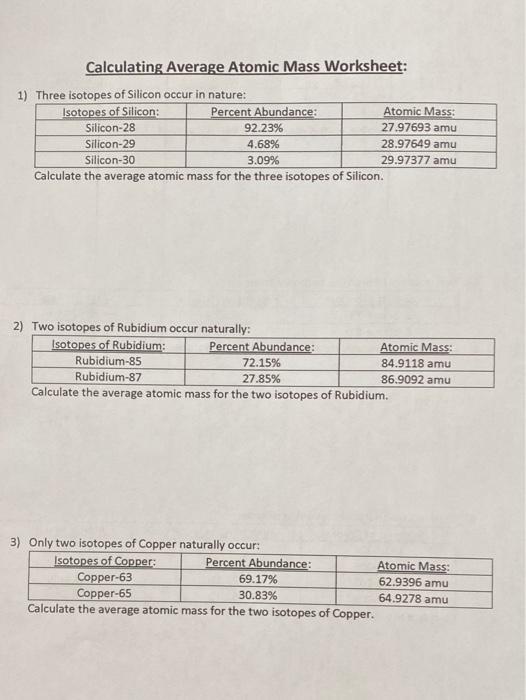
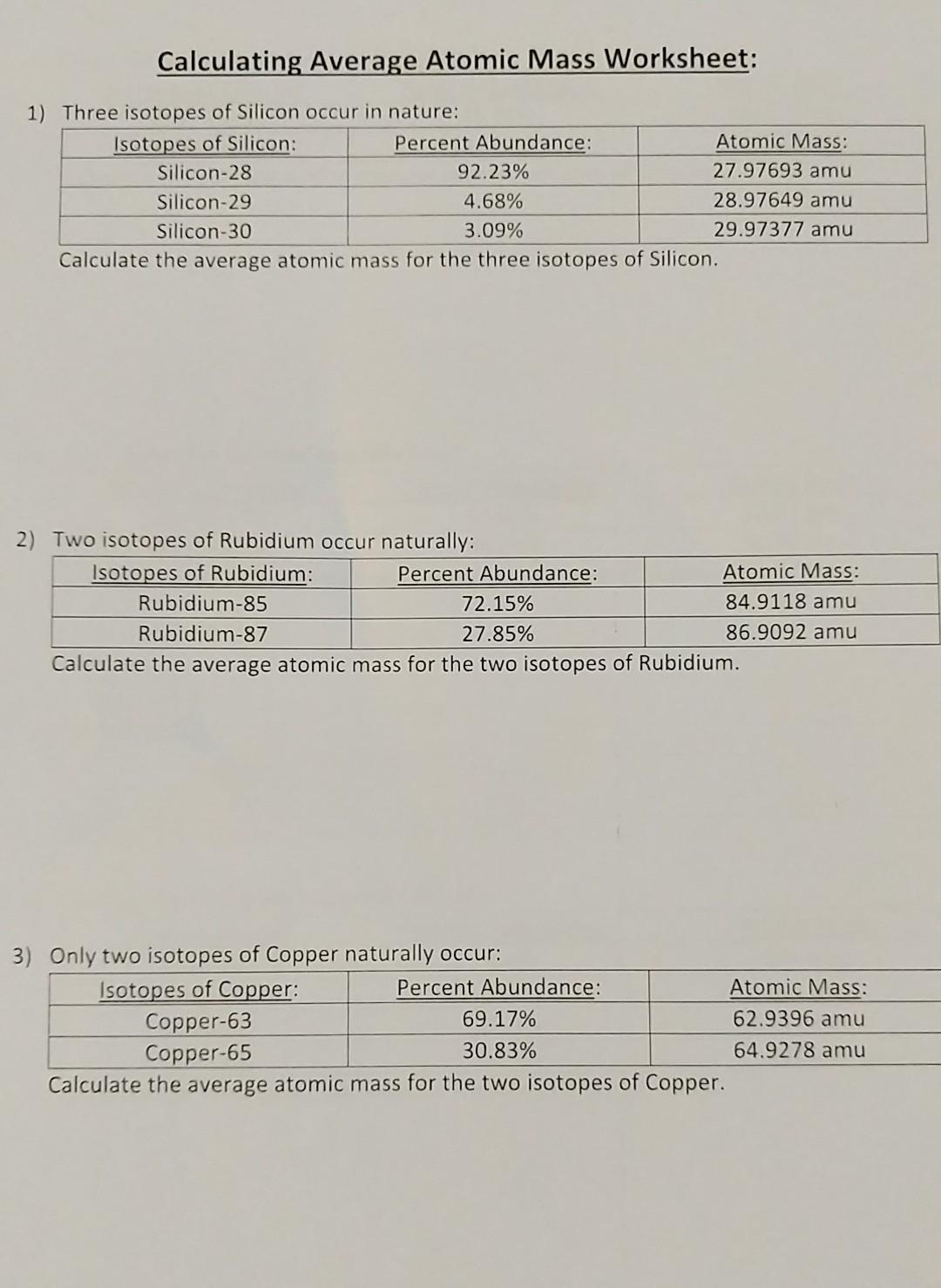
PDF Average Atomic Mass Practice Problems - scott.kyschools.us 2. Calculate the average atomic mass of lithium, which occurs as two isotopes that have the following atomic masses and abundances in nature: 6.017 u, 7.30% and 7.018 u, 92.70%. 3. 2Hydrogen is 99% 1H, 0.8% H, and 0.2% 3H. Calculate its average atomic mass. 4. Calculate the average atomic mass of magnesium using the following data for three Isotope & Average Atomic Mass Worksheet.docx - Name 28.97649 Si-30 3.09 29.97377Calculate the average atomic mass of Silicon. 3. Two isotopes of Copper occur naturally: Isotope Percent Abundance (%) Atomic Mass (amu) Cu-63 69.17 62.9396 Cu-65 30.83 64.9278 Calculate the average atomic mass of copper. 4. Lithium has two naturally occurring isotopes: lithium-6 and lithium-7. Isotopes and Average Weighted Atomic Mass | Pathways to Chemistry Isotopes and Average Weighted Atomic Mass. CHM161Isotopes and Average Weighted Atomic Mass. Answer Key. Back to Worksheets Atomic Number, Isotopes, and Atomic Mass Study Guide. Leave a Reply Cancel reply. Your email address will not be published. Required fields are marked * Comment *
Isotopes and average atomic mass worksheet. Isotopes And Average Atomic Mass Worksheets - K12 Workbook Displaying all worksheets related to - Isotopes And Average Atomic Mass. Worksheets are Chm 4 pal atomic mass student name, Livingston public schools lps home, Livingston public schools lps home, Honors chem atomic, Mayfield high school, Abundance of isotopes chemistry work 43 answer key, Isotopes and mass spectrometry, Unit 3. Lesson Worksheet:Percentage Isotopic Abundance | Nagwa In this worksheet, we will practice calculating percentage isotopic abundances from the relative atomic mass and isotopic masses. Q1: Chlorine has two stable isotopes, 3 5 C l and 3 7 C l , with atomic masses 34.9689 u and 36.9659 u respectively. The relative abundance of 3 7 C l in an average sample of chlorine is 3 7 3 5 C l C l = 0. 3 1 9 6. chemistry Test Worksheet: Isotopes Atomic Mass An atom that has 13 protons and 15 neutrons is an isotope of the element (1) nickel (2) silicon (3) aluminum (4) phosphorus 8 The weighted average of the atomic masses of the naturally occuring isotopes of an element is the (1) atomic mass of the element (2) atomic number of the element (3) mass number of each isotope PDF NAME Average Atomic Mass Worksheet: show all work. Calculate the average atomic mass. 35.46 amu 6) Copper used in electric wires comes in two flavors (isotopes): 63Cu and 65Cu. 63Cu has an atomic mass of 62.9298 amu and an abundance of 69.09%. The other isotope, 65Cu, has an abundance of 30.91%. The average atomic mass between these two isotopes is 63.546 amu. Calculate the actual atomic mass ...
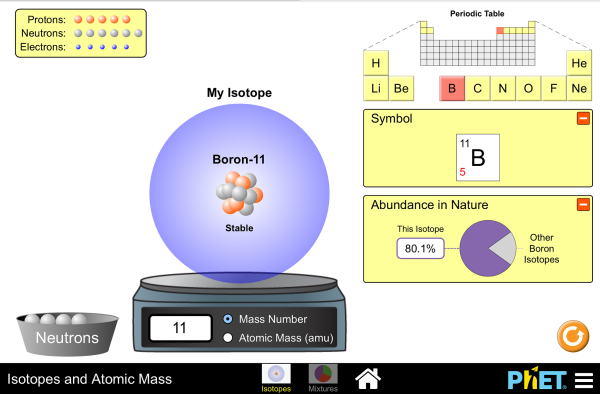
Isotope And Mass Spectrometry Worksheets - K12 Workbook *Click on Open button to open and print to worksheet. 1. AP WORKSHEET 01B: Isotopes and Mass Spectrometry 2. Isotopes and Mass Spectrometry 3. Isotope Practice Worksheet 4. WORKSHEET Atomic Structure and Mass Spectroscopy 5. An Introduction to Isotopic Calculations 6. Isotope and average atomic mass practice worksheet 7. Isotopes 8. 10.1.2. Isotopes And Average Atomic Mass Worksheet Answers isotopes-and-average-atomic-mass-worksheet-answers 1/59 Downloaded from intranet.vfymca.org on November 10, 2022 by guest Isotopes And Average Atomic Mass Worksheet Answers 9th Grade Chemistry Quick Study Guide & Workbook College Chemistry Quick Study Guide & Workbook A Level Chemistry Quick Study Guide & Workbook Science Atomic Number And Mass Worksheet Answers (Download Only) - edocs.utsa Isotope Practice Worksheet Name: 1. 2. D. 4. 13 12 Here are three isotopes of an element: ... Isotopes are atoms of the same element with a ... the . Ledermann Name 1. Determine the average atomic mass of the following mixtures of isotopes. 128 127 126 a. t, 17%- 3% I (sðf' 8) 197 198 ... Unbanked American households hit record low numbers in ... Isotopes and Atomic Mass - Isotopes | Atomic Mass - PhET Use the sim to learn about isotopes and how abundance relates to the average atomic mass of an element. Are all atoms of an element the same? How can you tell one isotope from another? Use the sim to learn about isotopes and how abundance relates to the average atomic mass of an element.
Calculating Average Atomic Mass Worksheet - Name ___Lisa ... - StuDocu The element Copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance and atomic masses are: 69% for mass of 62 30% for mass of 64. Calculate the average atomic mass of Copper. Show ALL work for full credit. 69/100 x 62 = 43. 30/100 x 64 = 19. ggghvhvhvhvhhv= 63 Isotopes and Weighted Average Atomic Mass Answer Key Study Guides for General Chemistry 1. 1. Matter and Measurement in Chemistry. 2. Atoms, Ions, and Molecules. 3. Chemical Reactions and Mass. 4. Reactions in Aqueous Solution. Alaisha Diaz Martinez - Isotopes and Average Atomic Mass Worksheet ... a o m cs u c u e atomic theory isotopes & average atomic massisotopes and average atomic mass practice worksheet 1.here are three isotopes of an element: 612c 613c 614c a.the element is: __________________ b.the number 6 refers to the number of _______________________ c.the numbers 12, 13, and 14 refer to the ________________________ d.how many … atomic structure and isotopes worksheet Calculating Average Atomic Mass Worksheets Answer Key . mass atomic worksheet average isotopes key answers calculating worksheets answer determination 10th unit excel db sponsored links. Atomic Structure And Isotopes Worksheet Answer Key + My PDF Collection 2021
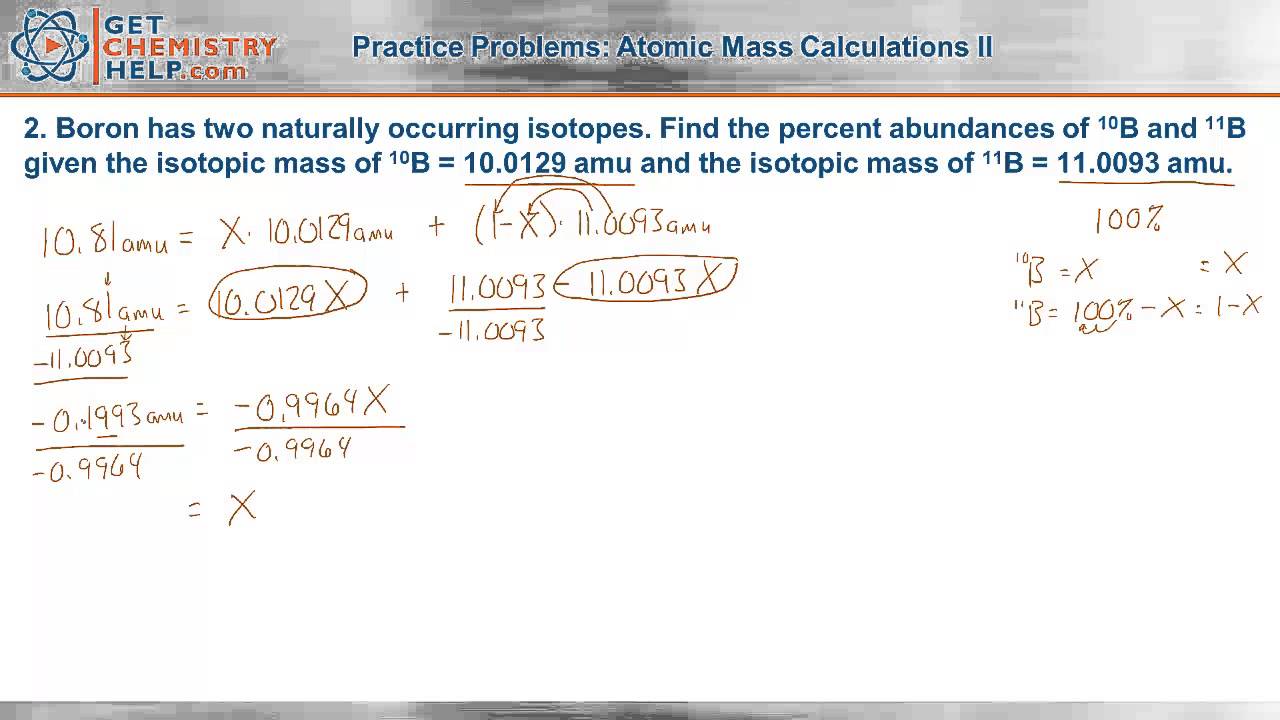
PDF Isotope Practice Worksheet 9. Iodine is 80% 127I, 17% 126I, and 3% 128I. Calculate the average atomic mass of iodine. 10. The natural abundance for boron isotopes is 19.9% 10B and 80.1% 11B . Calculate boron's atomic mass. 11. Hydrogen is 99% 1H, 0.8% 2H, and 0.2% 3H. Calculate its average atomic mass. 12. Rubidium is a soft, silvery-white metal that has two common ...
Isotopes and Average Atomic Mass - Study.com An element that has an atomic mass of 1.01 amu but is represented by both two different isotopes, one with a mass number of 1 and the other with 2, would have a much higher abundance of the ...
PDF Isotope Worksheet Answer Key - ISD 622 Isotope Practice Worksheet Name: 1. 2. D. 4. 13 12 Here are three isotopes of an element: a. The element is: ... Isotopes are atoms of the same element with a different number of ... Name 1. Determine the average atomic mass of the following mixtures of isotopes. 128 127 126 a. t, 17%- 3% I (sðf' 8) 197 198 19 q. 5 55 56 Fe, 85% 55)
PDF Isotopes and Atomic Mass Notes - gardencity.k12.ny.us Isotopes and Atomic Mass Notes - gardencity.k12.ny.us
Isotopes Worksheet - Google Docs Calculate the average atomic mass of strontium. 5. Titanium has five common isotopes: 46Ti (8.0%), 47Ti (7.8%), 48Ti (73.4%), 49Ti (5.5%), 50Ti (5.3%). What is the average atomic mass...
PDF Isotopes Average Atomic Mass - nyostrander.us 6. Weighted average of naturally occurring isotopes atomic mass 7. Total number of protons plus neutrons mass number 8. All electrons are in the lowest energy levels ground state 9. 1/12 the mass of a carbon-12 atom atomic mass unit (u) 10. How to solve for the number of neutrons mass number - atomic number 11.
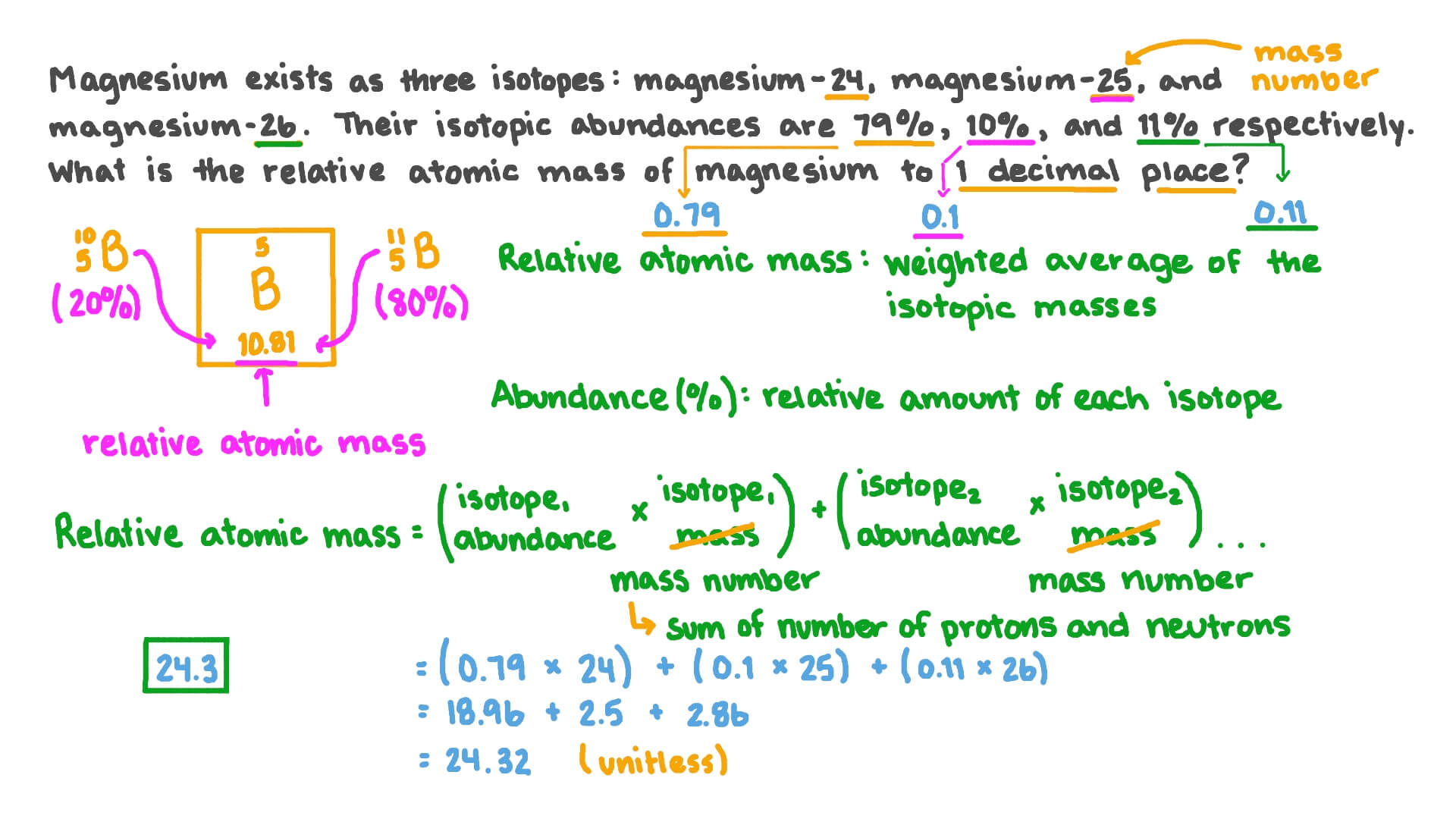
Worksheet_-_Average_Atomic_Mass_1.docx - Average Atomic... One of the isotopes has a mass number of 24. Another isotope has a mass number of 25 and is present 10.00% in nature. The third isotope has a mass number of 26 and is present 11.01% in nature. What is the average atomic mass of this element in amu and what element does this mass represent? 7. An element is a mixture of two isotopes. One isotope ...
Average Atomic Mass Practice Problems Quiz - Quizizz impossible to tell. Question 3. 900 seconds. Q. Calculate the average atomic mass of an element with the follow isotope information: 4.35% have a mass of 49.9461 amu, 83.79% have amass of 51.9405 amu, 9.50% have a mass of 52.9407 amu, and 2.36% have a mass of 53.9389 amu. answer choices. 51.99 amu.
PDF Isotope Practice Worksheet - Chemistry 7. Boron exists in two isotopes, boron-10 and boron-11. Based on the atomic mass, which isotope should be more abundant? Answer: The atomic mass of boron is 10.811; therefore, boron-11 is more abundant because the mass number is closer to the atomic mass. 8. Lithium-6 is 4% abundant and lithium-7 is 96% abundant. What is the average mass of ...
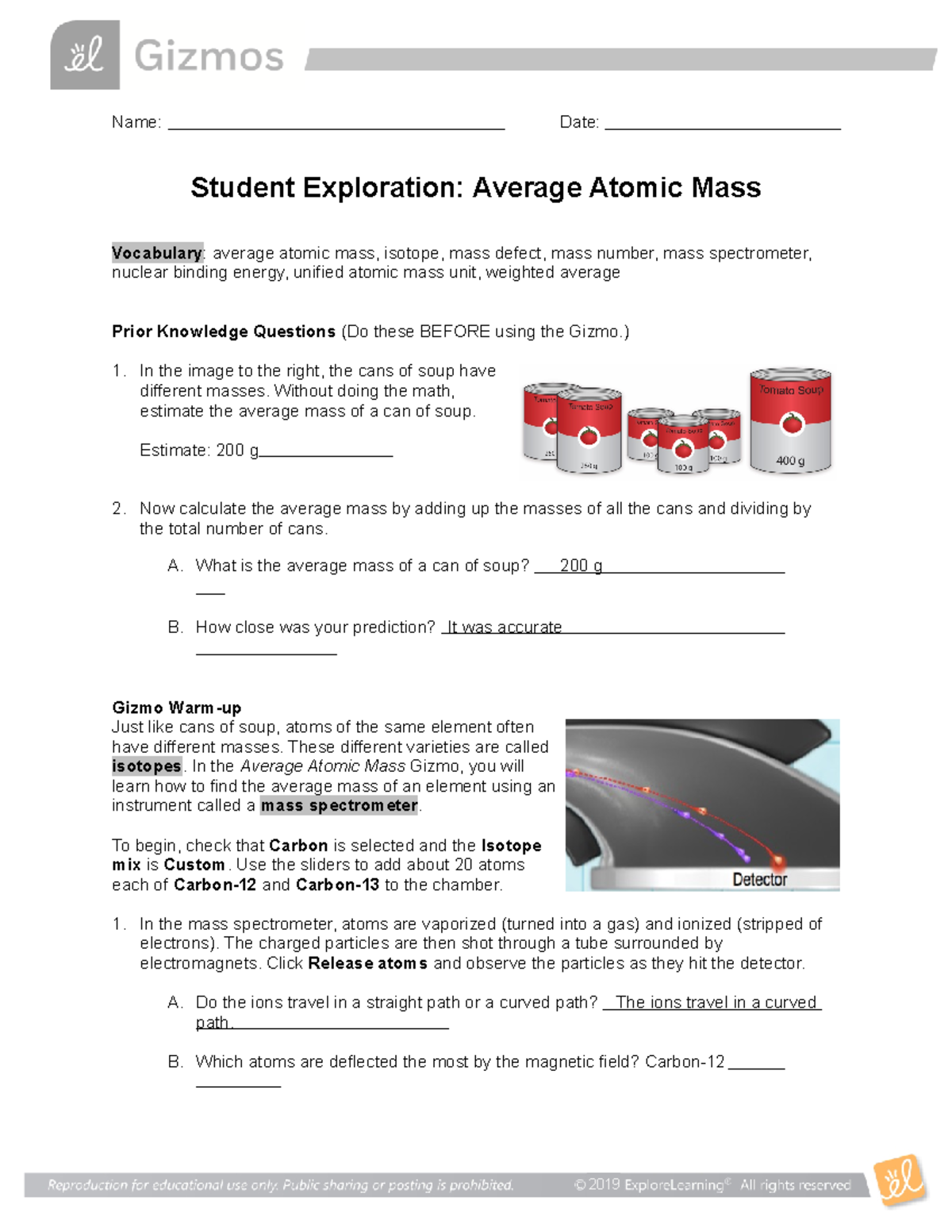
Isotopes And Average Atomic Mass Worksheet Answers varieties are called isotopes. In the Average Atomic Mass Gizmo, ... Isotope Worksheet Answer Key - ISD 622 Isotope Practice Worksheet Name: 1. 2. D. 4. 13 12 Here are three isotopes of an element: a. The element is: b. The number 6 refers to the c. The numbers 12, 13, and 14 refer to the ... Determine the average atomic mass of the following
Isotopes and Average Atomic Mass - Quiz & Worksheet Isotopes and Average Atomic Mass - Quiz & Worksheet Video Quiz Course Try it risk-free for 30 days Instructions: Choose an answer and hit 'next'. You will receive your score and answers at...
Isotopes and Average Weighted Atomic Mass | Pathways to Chemistry Isotopes and Average Weighted Atomic Mass. CHM161Isotopes and Average Weighted Atomic Mass. Answer Key. Back to Worksheets Atomic Number, Isotopes, and Atomic Mass Study Guide. Leave a Reply Cancel reply. Your email address will not be published. Required fields are marked * Comment *
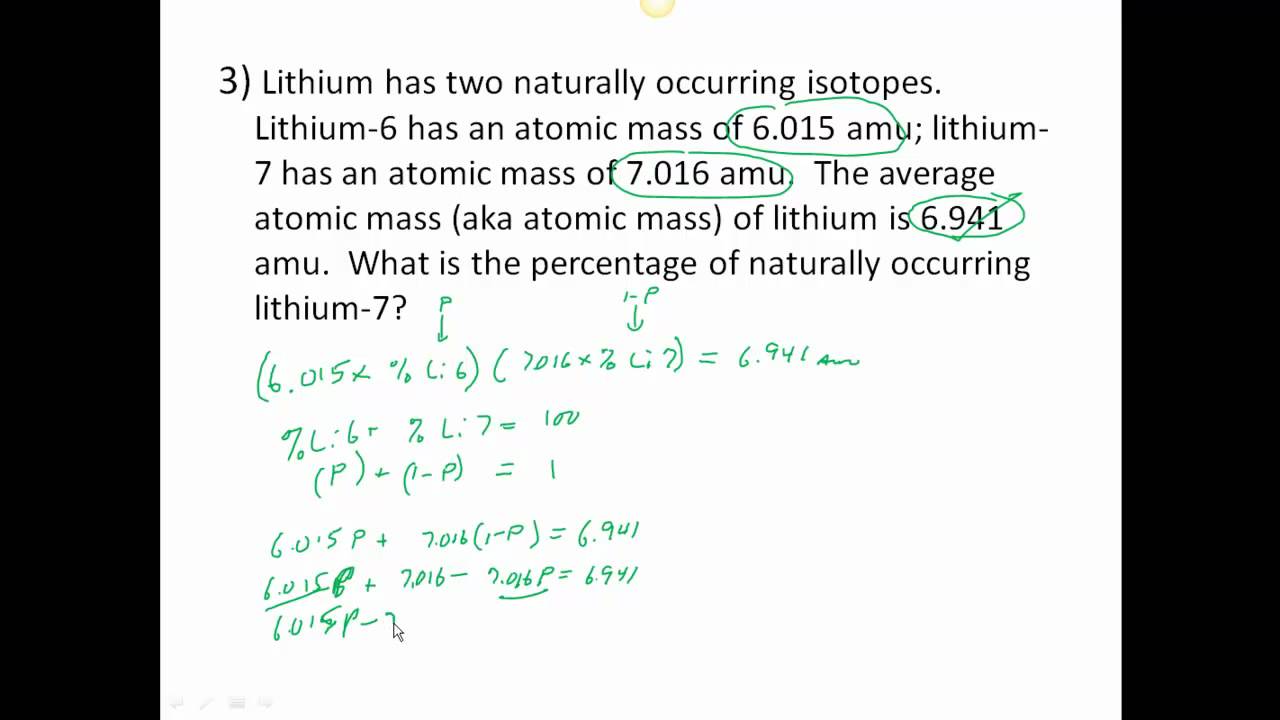
Isotope & Average Atomic Mass Worksheet.docx - Name 28.97649 Si-30 3.09 29.97377Calculate the average atomic mass of Silicon. 3. Two isotopes of Copper occur naturally: Isotope Percent Abundance (%) Atomic Mass (amu) Cu-63 69.17 62.9396 Cu-65 30.83 64.9278 Calculate the average atomic mass of copper. 4. Lithium has two naturally occurring isotopes: lithium-6 and lithium-7.
PDF Average Atomic Mass Practice Problems - scott.kyschools.us 2. Calculate the average atomic mass of lithium, which occurs as two isotopes that have the following atomic masses and abundances in nature: 6.017 u, 7.30% and 7.018 u, 92.70%. 3. 2Hydrogen is 99% 1H, 0.8% H, and 0.2% 3H. Calculate its average atomic mass. 4. Calculate the average atomic mass of magnesium using the following data for three




























0 Response to "45 isotopes and average atomic mass worksheet"
Post a Comment