38 redox reactions worksheet with answers
Balancing redox reactions in basic solution. Answers to practice problems 1. Mg mg2 2e d. Balancing redox reactions worksheet 1 balance each redox reaction in. Balancing redox reactions chem 1a b steps for balancing redox reactions with the reaction method. In the reaction mg cl2 mgcl2 the correct half reaction for the oxidation that occurs is a.
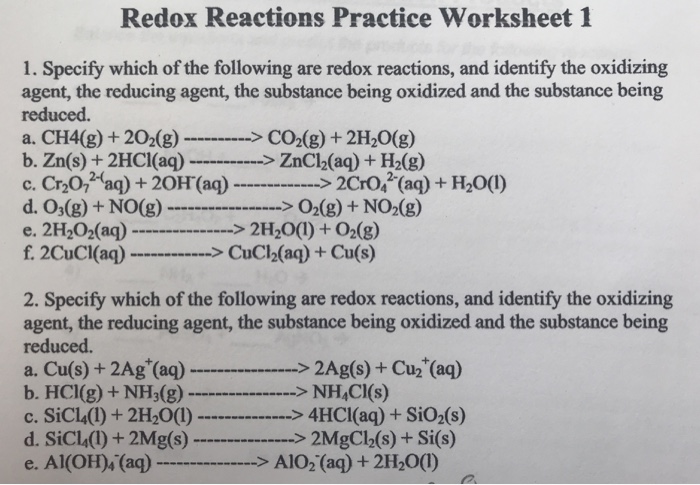
Write balanced equations for the following redox reactions: a. 2 NaBr + Cl 2 2 NaCl + Br 2 b. Fe 2 O 3 + 3 CO 2 Fe + 3 CO 2 in acidic solution c. 5 CO + I 2 O 5 5 CO 2 + I 2 in basic solution ; Write balanced equations for the following reactions: a. Cr(OH) 3 + Br 2 CrO 4 2-+ Br-in basic solution 10 OH-+ 2 Cr(OH) 3 + 3 Br 2 2 CrO 4 2-+ 8 H 2 O + 6 Br-b.
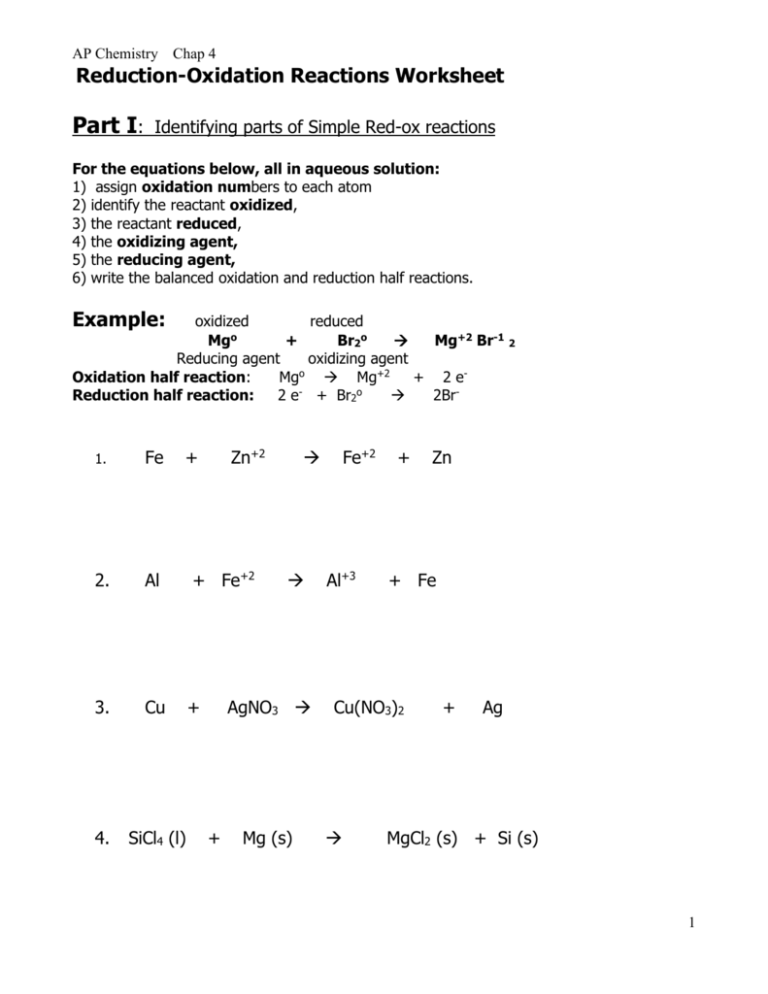
Oxidation Reduction Worksheet. Determine the oxidation number of each atom in the following substances. NF3 N +3 F -1 K2CO3 K +1 C 4 O -2 c. NO3- N____+5_____ O____-2_____ HIO4 H +1 I +7 O -2 For the following balanced redox reaction answer the following questions. 2 Fe+2(aq) + H2O2(aq) ( 2Fe+3(aq) + 2 OH-1(aq) What is the oxidation state of ...
Redox reactions worksheet with answers
May 08, 2013 · 14. Given the reaction: 2Na+2H2O !2Na+ +2OH +H2 Which substance is oxidized? A. H2 B. H+ C. Na D. Na+ 15. Which change occurs when an Sn2+ ion is oxidized? A. Two electrons are lost. B. Two electrons are gained. C. Two protons are lost. D. Two protons are gained. 16. A redox reaction always involves A. a change in oxidation number B. a change in phase
Mno 2 mn 2o 3 balance each redox reaction in acid solution using the half reaction method. Redox reactions worksheet answers. A redox reaction always involves a. Oxidation is associated with electron loss helpful mnemonic. H 2o 2 cr 2o 7 2 o 2 cr 3 9. Determination of activity of some metals by reaction with hydrogen ion doc 28 kb redox ...
Questions 12-13: Balancing redox reaction equations is a skill which combines chemical knowledge, common sense, and intuition. There are many methods available for balancing redox reactions, A number have been brought to your attention including the one that follows. Choose a method and complete questions 12-13.
Redox reactions worksheet with answers.
Recognizing redox reactions worksheet answers. 3mg n2 mg3n2 5. Balance each of the following half cell reactions. Suited for student in y10 and y11. In the reaction mg cl2 mgcl2 the correct half reaction for the oxidation that occurs is a. Redox reactions are a chemical reaction in which electrons are exchanged through oxidation and reduction.
Balancing REDOX Reactions: Learn and Practice Reduction-Oxidation reactions (or REDOX reactions) occur when the chemical species involved in the reactions gain and lose electrons. Oxidation and reduction occur simultaneously in order to conserve charge. We can “see” these changes if we assign oxidation numbers to the reactants and products.
Balancing Redox Reactions Worksheet 1 Balance each redox reaction in . acid. solution. Mn 2+ + BiO3 -Æ MnO4 -+ Bi 3+ MnO4 -+ S2O3 2- Æ S4O6 2- + Mn 2+ ClO3 - + Cl - Æ Cl2 + ClO2 . P + Cu 2+ Æ Cu + H2PO4 -PH3 + I2 Æ H3PO2 -+ I -NO2 Æ NO3 -+ NO . Basic Solutions . MnO4 -+ C2O4 2- Æ MnO2 + CO2 . ClO2 Æ ClO2 -+ ClO3 -
Practice Problems: Redox Reactions. Determine the oxidation number of the elements in each of the following compounds: a. H 2 CO 3 b. N 2 c. Zn(OH) 4 2-d. NO 2-e. LiH f. Fe 3 O 4 Hint; Identify the species being oxidized and reduced in each of the following reactions: a. Cr + + Sn 4+ Cr 3+ + Sn 2+ b. 3 Hg 2+ + 2 Fe (s) 3 Hg 2 + 2 Fe 3+ c. 2 As ...
Worksheet # 5 Balancing Redox Reactions in Acid and Basic Solution Balance each half reaction in basic solution. 4. Cr 2O 7 2 - → Cr3+ 5. NO → NO 3-6. SO 4 2- → SO 2 7. MnO 2 → Mn 2O 3 Balance each redox reaction in acid solution using the half reaction method. 8. H 2O 2 + Cr 2O 7 2- → O 2 + Cr 3+ 9. TeO 3 2-+ N 2O 4 → Te + NO 3-10. ReO 4
Predicting redox reactions using the half reaction table 1. Redox reactions worksheet. Cr 2o 7 2 cr3 5. Mn 2 bio3 æ mno4 bi 3 mno4 s2o3 2 æ s4o6 2 mn 2. In the reaction 2k cl2 2kcl the species. In the reaction al0 cr3 al3 cr0 the reducing agent is a. Ws 4 balancing redox reactions. 3mg n2 mg3n2 5. 2cs br2 2csbr 4. 5 2 customer reviews.
Worksheet 25 - Oxidation/Reduction Reactions Oxidation number rules: Elements have an oxidation number of 0 Group I and II – In addition to the elemental oxidation state of 0, Group I has an oxidation state of +1 and Group II has an oxidation state of +2. Hydrogen –usually +1, except when bonded to Group I or Group II, when it forms hydrides, -1. ...
Redox reactions practice problems determining oxidation numbers worksheet answers. For example the oxidation number of the oxygen in the oxide ion o 2 is 2. 1 This problem poses interesting problems especially with the Cl. Fe HCl —. In which substance is the oxidation number of. The oxidation number of hydrogen in a compound is 1 4.
Redox Reactions Answer Key Redox Reactions CHEM 10 Review Worksheet The questions on this worksheet are both Chem 10 and Chem 11 level questions. The reaction that takes place in a chemical cell is best classi ed as A. Redox Reactions Worksheet Pdf Printable worksheets are a valuable lecture room tool. Oxidizedreducing agent O0 to O2-.
admin November 18, 2019. Some of the worksheets below are Redox Reactions Worksheets, useful trick to help identify oxidation and reduction, step by step guide to balance any Redox Equations, explanation of Oxidation, reduction, oxidizing agent, reducing agent and rules for assigning an oxidation number, …. Once you find your worksheet (s ...
25 Redox Review Worksheet Answers Scientific Notation Word Problems Redox Reactions Word Problem Worksheets . Pin By Quinna On Delete Igka Homan In 2021 Chemistry Redox Reactions Oxidation State . Half Reaction Of Wo2 To Wo3 Please Explain Yahoo Answers Yahoo Answers Answers Reactions .
Redox reaction worksheet. Subject: Chemistry. Age range: 14-16. Resource type: Worksheet/Activity. 4.7 3 reviews. ktbax263. 4.671428571428572 11 reviews. Last updated. ... This is excellent, a really useful worksheet. Would love to have the answers too if possible next time! Thanks again! Show replies
WS # 4 Balancing Redox Reactions . Balance each of the following half-cell reactions. (In each case assume that the reaction takes place in an ACIDIC solution.) Also, state whether the reaction is oxidation or reduction. 1.
Worksheet 1 - Oxidation/Reduction Reactions Oxidation number rules: Elements have an oxidation number of 0 Group I and II – In addition to the elemental oxidation state of 0, Group I has an oxidation state of +1 and Group II has an oxidation state of +2. Hydrogen –usually +1, except when bonded to Group I or Group II, when it forms hydrides, -1. ...
Balancing redox reactions worksheet with answers pdf. Zn no 3 zn2 nh4 3. In the first case you separate out the oxidation and reduction half reaction and in the second case you do it all at once. Be sure the reaction is redox look at the oxidation numbers for the atoms in the reaction. Teo 3 2 n 2o 4 te no 3 10.
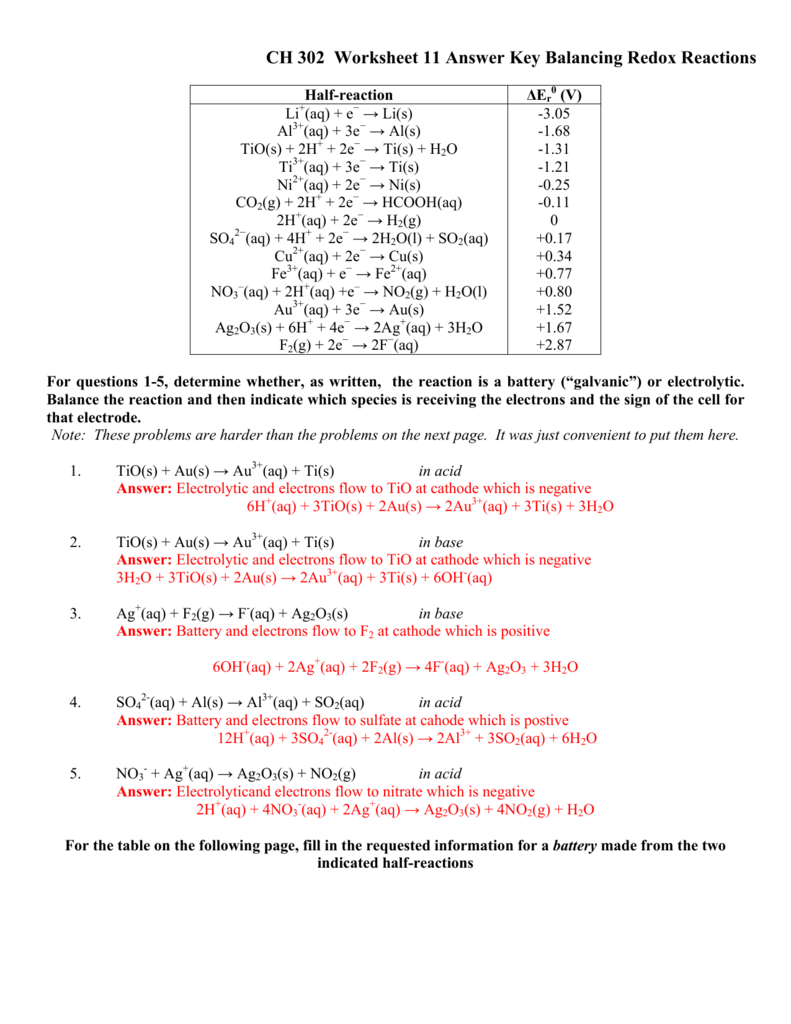
Balancing Redox Equations Method 2: Half-reaction method 1. Divide the skeleton reaction into two half-reactions, each of which contains the oxidized and reduced forms of one of the species 2. Balance the atoms and charges in each half-reaction - Atoms are balanced in order: atoms other than O and H, then O, then H
Redox Reactions Worksheet With Answers. Cr oh 3 br 2 cro 4 2 br in basic solution 10 oh 2 cr oh 3 3 br 2 2 cro 4 2 8 h 2 o. What is the oxidation number of carbon in NaHCO3. Divide the skeleton reaction into two half reactions each of which contains the oxidized and reduced forms of one of the species 2. MnO 2 Mn 2O 3 Balance each redox ...
! 211!! ThehalfJreaction!method!involves!balancing!the!oxidation!reaction!as!if!it! wereanisolatedreaction.Thenthereductionhalf Jreaction!isbalancedasifit!were
Chapter 20 Worksheet: Redox I. Determine what is oxidized and what is reduced in each reaction. Identify the oxidizing agent and the reducing agent, also. 1. 2Sr + O2 2SrO 2. 2Li + S Li2S 3. 2Cs + Br2 2CsBr 4. 3Mg + N2 Mg3N2 5. 4Fe + 3O2 2Fe2O3 6. Cl2 + 2NaBr 2NaCl + Br2 7. Si + 2F2 SiF4 8. 2Ca + O2 2CaO 9.
Redox reactions answer key determine the oxidation number of the elements in each of the following compounds. In the reaction 2k cl2 2kcl the species. Balance the reaction and indicate which reactant is oxidized and which reactant is being reduced. Identify the species being oxidized and reduced in each of the. Redox practice worksheet name.
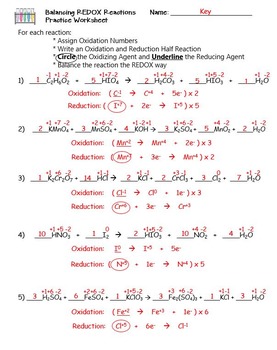
For each reaction below, identify the atom oxidized, the atom reduced, the oxidizing agent, and the reducing agent. 1) Mg + 2HCl ( MgCl2 + H2. 2) 2Fe + 3V2O3 ( Fe2O3 + 6VO. 3) 2KMnO4 + 5KNO2 + 3H2SO4 ( 2MnSO4 + 3H2O + 5KNO3 + K2SO4. ... Oxidation Reduction Worksheet Answers ...
reaction and reduction half-reaction, identify the oxidizing and reducing agents and any spectator ions. Half - Reactions Homework ... Balance the following redox reactions by the half-reaction method, rewriting the balanced equations below the given unbalanced equation. Show your work below each reaction and
Questions pertaining to redox reactions. 1 in combination with nonmetals o n. Cr 2o 7 2 cr3 5. No no 3 6. Balancing redox reactions worksheet 1. Clo3 cl æ cl2 clo2. 1 in all compounds 2. A change in phase. H 2o 2 cr 2o 7 2 o 2 cr 3 9. Choose a method and complete questions 12 13.




























0 Response to "38 redox reactions worksheet with answers"
Post a Comment