42 ionic bonding worksheet answers page 38
Simple Ionic Coumpound Worksheet.pdf View Download 49k: v. 2 : Feb 25, 2012, 8:49 PM: J Idone: Ċ: SimpleIonWS.pdf View Download: Naming Ionic Compounds with Simple Metals Worksheet 33k: v. 3 : Apr 11, 2012, 6:20 PM: J Idone: Ċ: Type II Binary Ionic Compounds contain Transition metals.pdf View Download: Naming Ionic Compunds with Transition ...
Chemical Bonding. Ionic Bonds: atoms give up or gain e- and. are attracted to each other by. coulombic attraction . Na. Na+. Cl. Cl- loses e- gains e- Na+ + Cl- NaCl . K+ + NO. 3 -KNO. 3 ionic compounds = salts. where NO. 3 - is a polyatomic ion: a charged group of . atoms that stay together . ionic bonds: M + NM. cation + anion
Naming Mixed Ionic and Covalent - Answers Name the following compounds. Remember, they may be either ionic or covalent compounds, so make sure you use the right naming method! 1) NaF sodium fluoride 2) NF 3 nitrogen trifluoride 3) Li 2O lithium oxide 4) Al 2S 3 aluminum sulfide 5) MgSO 4 magnesium sulfate 6) SiH 4 silicon tetrahydride 7) KNO 3 ...

Ionic bonding worksheet answers page 38
Ionic Bonding Worksheets Answers Page 38. November 6, 2019. Sponsored Links. Mixed Coins Worksheets. Esl Printable Worksheets For Adults. Common Multiple Worksheets. Bones Of The Body Worksheets. Compounds And Molecules Worksheets. Note Naming Worksheets Treble Clef. Adjacent Angles Worksheets. Hindi Alphabet Practice Worksheets. Wrain.
Worksheet 5.1 Writing and Naming Ionic Compounds with Polyatomic Ions and Transition ... 38. LiBr lithium bromide 39. SrO strontium oxide 40. PbCl4 lead (IV) chloride 41. Cs2S cesium sulfide ... Answer Key Worksheet 5.1 Naming and Writing Ionic Compounds with Polyatomic Ions
38) CdSO 3 _____ 39) Cu(NO 2) 2 ... Choose the one alternative that best completes the statement or answers the question. 81) Which of the following pairs of elements would most likely form a ionic compound? ... Solutions for the Naming Ionic Compounds Practice Worksheet 1) ammonium chloride 2) iron (III) nitrate 3) titanium (III) bromide
Ionic bonding worksheet answers page 38.
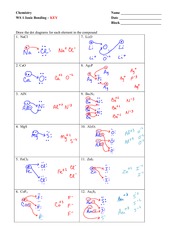
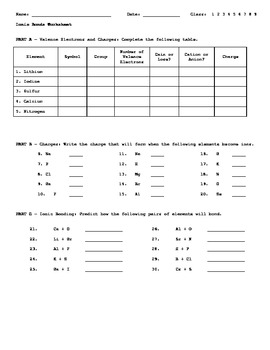
Ionic Bonding Worksheet. Complete the chart for each element. Element # of Protons # of Electrons # of Valence Electrons Oxidation Number Sodium 11 11 1 +1 Calcium 20 20 2 +2 Aluminum 13 13 3 +3 Chlorine 17 17 7 -1 Beryllium 4 4 2 +2 Fluorine 9 9 7 -1 Lithium 3 3 1 +1 Iodine 53 53 7 -1 Oxygen 8 8 6 -2 Potassium 19 19 1 +1 Magnesium 12 12 2 +2 Phosphorus 15 15 5 -3
Nomenclature #2: Polyatomic Ionic Compounds 1. Name the following compounds (include Roman Numerals when necessary): Na 2SO 4 sodium sulfate AℓPO 4 aluminum phosphate Aℓ (C ℓO 4) 3 aluminum perchlorate AsPO 3 arsenic (III) phosphite Ni(OH) 3 nickel (III) hydroxide AgBrO 3 silver bromate Pb(IO 3) 2 lead (II) iodate K 3P potassium phosphide HgCN mercury (I) cyanide Mg(IO 4)
Chemical Bonding Worksheet Ionic Bond between a Metal and Non-Metal (M + NM) Covalent Bond between a Non-Metal and Non-Metal (NM + NM) Metallic Bond between a Metal and Metal (M+ M) Directions: 1. Determine if the elements in the following compounds are metals or non-metals 2.
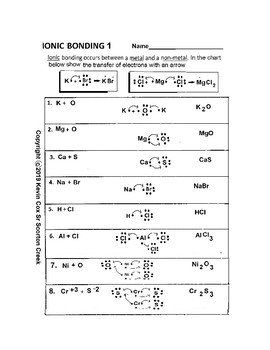
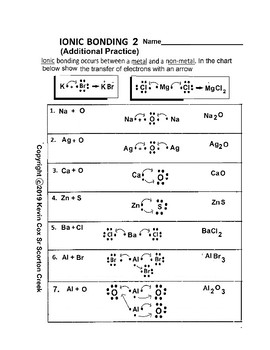
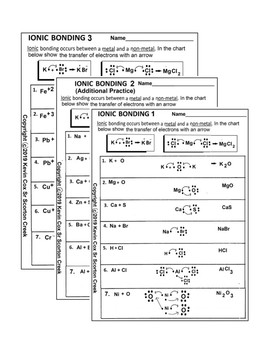
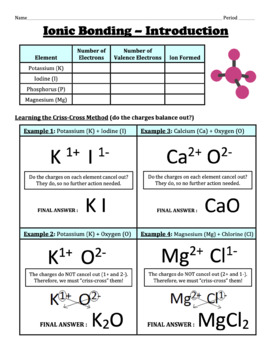
Ionic BOnding Diagrams. Today we discussed ionic bonding diagrams and reviewed how the charge of the ions helps hold the bond together. Students we given a worksheet and we worked through the first 6 together, applying what we knew about the electrons to figure out the ions formed. Students will turn in this sheet on WEDNESDAY to be graded as ...
Ionic Compound Naming - Chilton Honors Chemistry Chemical Formula Writing Worksheet Solutions Write chemical formulas for the compounds in each box. The names are found by finding the intersection between the cations and anions. Example: The first box is the
Naming binary molecular compounds Worksheet#3. Binary molecular compounds are made from a combination of 2 different atoms, or in the case of diatomic molecules one kind of atom, ie. Br I N Cl H O F. When naming a binary molecular compound you need to use prefixes 1 = mono 6 = hexa 2 = di 7 = hepta 3 = tri 8 = octa 4 = tetra 9 = nona
Chem Worksheet 8-2 Name An ionic compound is a combination of oppositely charged ions. Ionic compounds generally contain a metal bonded to a non-metal (or non-metals). When naming ionic compounds we simply name the cation (the positive ion) then the anion (the negative ion). The cations generally retain the name of the element. so Na' is named ...
SNC2D Chem02a Answers - Binary Ionic Compounds.pdf View Download 38k: v. 2 : Dec 5, 2013, 6:10 PM: Jeffrey Warner [Staff] Ċ: SNC2D Chem02b Answers - Multivalent Metals.pdf View Download 43k: v. 2 : Dec 5, 2013, 6:10 PM: Jeffrey Warner [Staff] Ċ: SNC2D Chem02c Answers to Ionic Compounds Worksheet.pdf View Download 31k: v. 2 : Dec 5, 2013, 6:10 ...
Ionic compounds conduct electricity • For a material to conduct an electric current, there must be charged particles that can move. • Ionic compounds in a liquid state or dissolved in water can conduct electricity > Ions are free to move • An aqueous solution of an ionic compound that conducts electricity is called an electrolyte.
Nomenclature Worksheet 2: Simple Binary Ionic Compounds Please complete the following table: Formula of Ionic Compound s 2. 3. 4. 5. 6. 8. 9. Name of Ionic Compound
1 Chemical bonds, Ionic, Covalent, Metallic 2 2 24 3 38 4 How bond + structure relate to props 50 5 63 6 82 7 Structure + bonding carbon 97 8 122 9 131 10 Bulk + surface properties inc nano particles 145 11 170 12 180
Ionic Bonding & Ionic Compounds Multiple Choice Review ... 38)Aluminum reacts with a certain nonmetallic element to form a compound with the general formula Al 2 X ... www.njctl.org Chemistry Ionic Bonding ANSWERS: 1) C 2) D 3) D 4) E 5) C 6) C 7) A 8) C 9) D 10)E 11)E 12)A 13)E 14)A 15)B 16)B 17)B 18)E 19)B ...
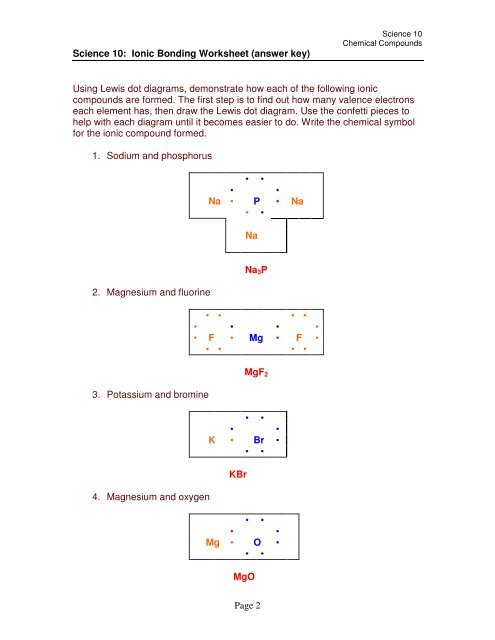
Naming Chemical Compounds - Answers Name the following ionic compounds: 1) NaBr sodium bromide 2) CaO calcium oxide 3) Li 2 S lithium sulfide 4) MgBr 2 magnesium bromide 5) Be(OH) 2 beryllium hydroxide Write the formulas for the following ionic compounds: 6) potassium iodide KI 7) magnesium oxide MgO 8) aluminum chloride AlCl 3
Nomenclature Worksheet 6: Binary Covalent Compounds Please complete the follo#ng table: Name of CovalentCompound 1. carbon dioxide 2. phosphorus triiodide 3, sulfur dichloride 4, nitrogen trifluoride i 5. dioxygen difluodde Formula of Cova~entCornpound 16. N2F4 7. SCI~ 8. ClF~ 9. Si02 10.P4010
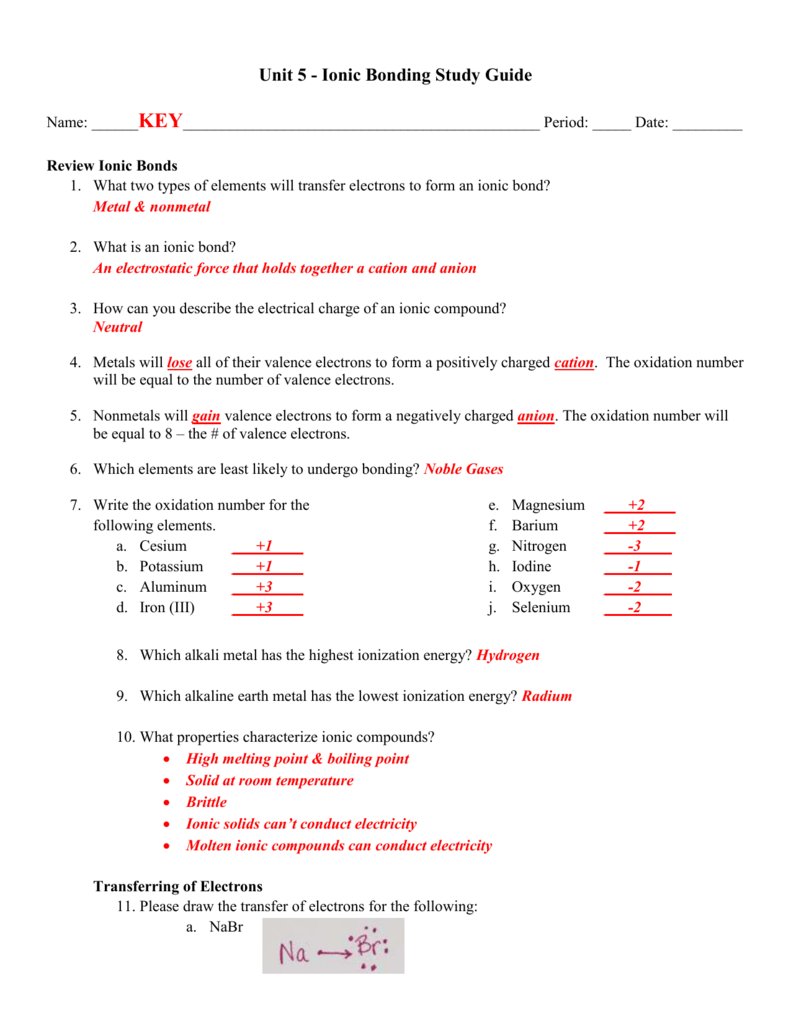
3. What happens to the electrons in an ionic bond? are --íran9fered 4. What is a binary compound? Give an example and a non-example of a binary compound. con+Qins z el 5. What are the five properties of an ionic compound? non-ex : g NC) Scon9 bonds) i zed or Chary e 6. What type of elements form an ionic bond? 7. What force holds the ionic ...
38) molybdenum sulfate _____ 39) platinum (II) sulfide _____ 40) ammonium sulfate _____ Mixed Ionic/Covalent Compound Naming For each of the following questions, determine whether the compound is ionic or covalent and name it appropriately. 1) Na 2 CO 3
Ionic Compound Formula Writing Worksheet. Write chemical formulas for the compounds in each box. The names are found by finding the intersection between the cations and anions. Example: The first box is the intersection between the "zinc" cation and the "chloride" anion, so you should write "ZnCl. 2 ", as shown.
Naming Ionic Compounds Practice Worksheet - Solutions Name the following ionic compounds: 1) NH4Cl ammonium chloride 2) Fe(NO3)3 iron (III) nitrate 3) TiBr3 titanium (III) bromide 4) Cu3P copper (I) phosphide 5) SnSe2 tin (IV) selenide 6) GaAs gallium arsenide
II. Name the following Covalent Compounds:! 1. CO2 carbon dioxide ! 2. CO carbon monoxide ! 3. SO2 sulfur dioxide ! 4. SO3 sulfur trioxide ! 5. N 2O dinitrogen monoxide ! 6. NO nitrogen monoxide ! 7. N2O3 dinitrogen trioxide ! 8. NO2 nitrogen dioxide ! 9. 19.N2O4 2 dinitrogen tetroxide ! 10. N2O5 dinitrogen pentoxide 11. PCl3 phosphorous ...
Bonding Worksheet #1: Introduction to Ionic Bonds. ... Determine if it is an ionic bond or a covalent bond. Show the work and the final answer. Remember: Covalent bonds form between two nonmetals that share electrons. Ionic bonds are formed between a metal and a nonmetal that completely transfer electrons. ... 38. The strongest of the above ...
Created Date: 20130425213024Z
Polyatomic ions worksheet answer key. OsIO33 Os3 3. ... Names and formulas for ionic compounds worksheet answers - Learning about the actual value of cash is among the main training kids of. ... Ion number of protons number of electrons co 2 27 25 co 3 27 24 cl 1 17 18 k 1 19 18 s 2 16 18 sr 2 38 36 al 3 13 10 p 3 15 18 how many protons ...
COVALENT BONDING Name Covalent bonding occurs when two or more nonmetals share electrons. attempting to attain a stable octet of electrons at least part of the time. For example: Note that hydrogen Is content with 2, not 8. electrons. Show how covalent bonding occurs in each of the following pairs of atoms. Atoms may
Ionic Bonding. Valence Electrons. Electron Dot Structure. Octet Rule. Cation. Electrons on the outermost energy level of an atom. a notation that depicts valence electrons as dots around the a…. States that atoms lose, gain or share electrons in order to ac….


























0 Response to "42 ionic bonding worksheet answers page 38"
Post a Comment