38 atoms protons neutrons electrons worksheet
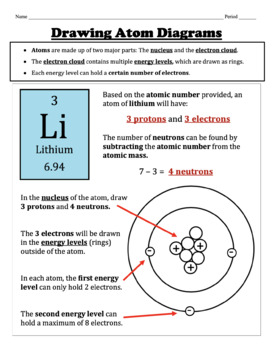
Some elements, such as carbon, potassium, and uranium, have multiple naturally-occurring isotopes. Isotopes are defined first by their element and then by the sum of the protons and neutrons present. Carbon-12 (or 12 C) contains six protons, six neutrons, and six electrons; therefore, it has a mass number of 12 amu (six protons and six neutrons). Iupac periodic table nuclide notation how many protons neutrons and electrons in atoms atoms and the periodic table. This isotope of carbon has 6 protons and 6 neutrons. For hydrogen the atomic number is 1 because there is one proton and no neutrons. 5 protons electrons 6 neutrons 108 rounds up to 11 is the atomic mass.
Dec 18, 2021 · Protons Neutrons and Electrons Worksheet. Electrons have a negative charge. Electrons have a negative charge. Worksheet 2 calculating protons electrons and neutrons element symbol atomic number atomic mass protons electrons neutrons h 1 1 2 2 li 7 be 4 9 5 5 6 c 12 7 14 o 16 8 f 9 ne 20 11 23 12 12 al 14 15 31 32 cl 17.

Atoms protons neutrons electrons worksheet
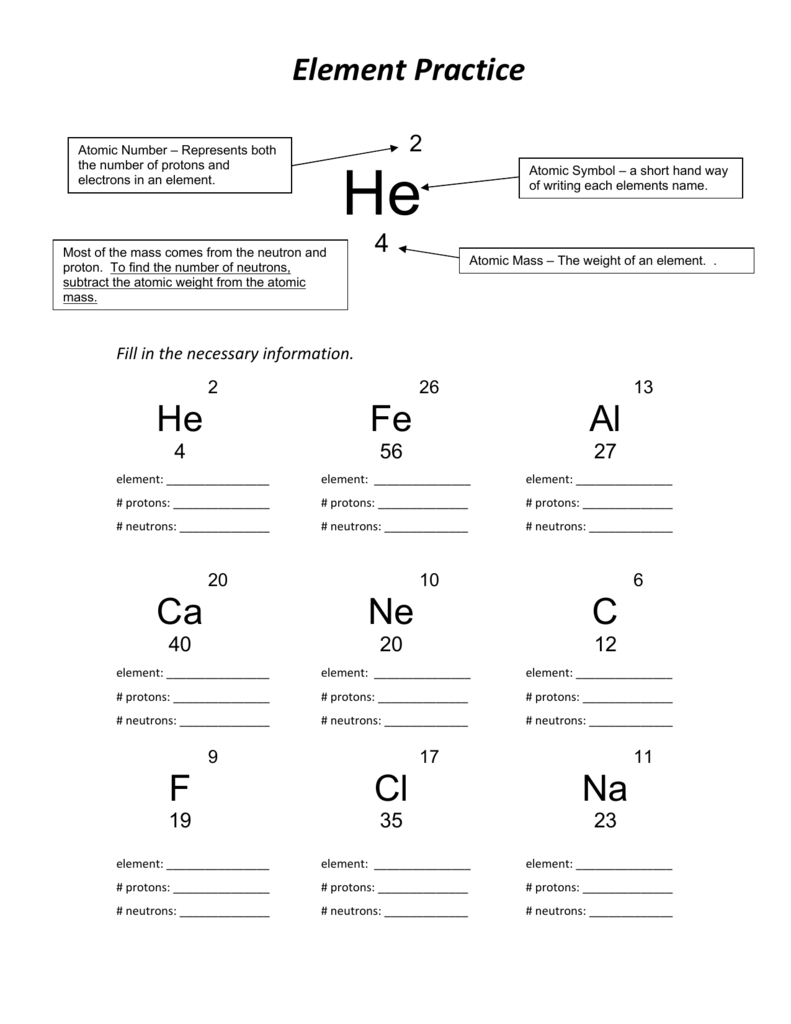
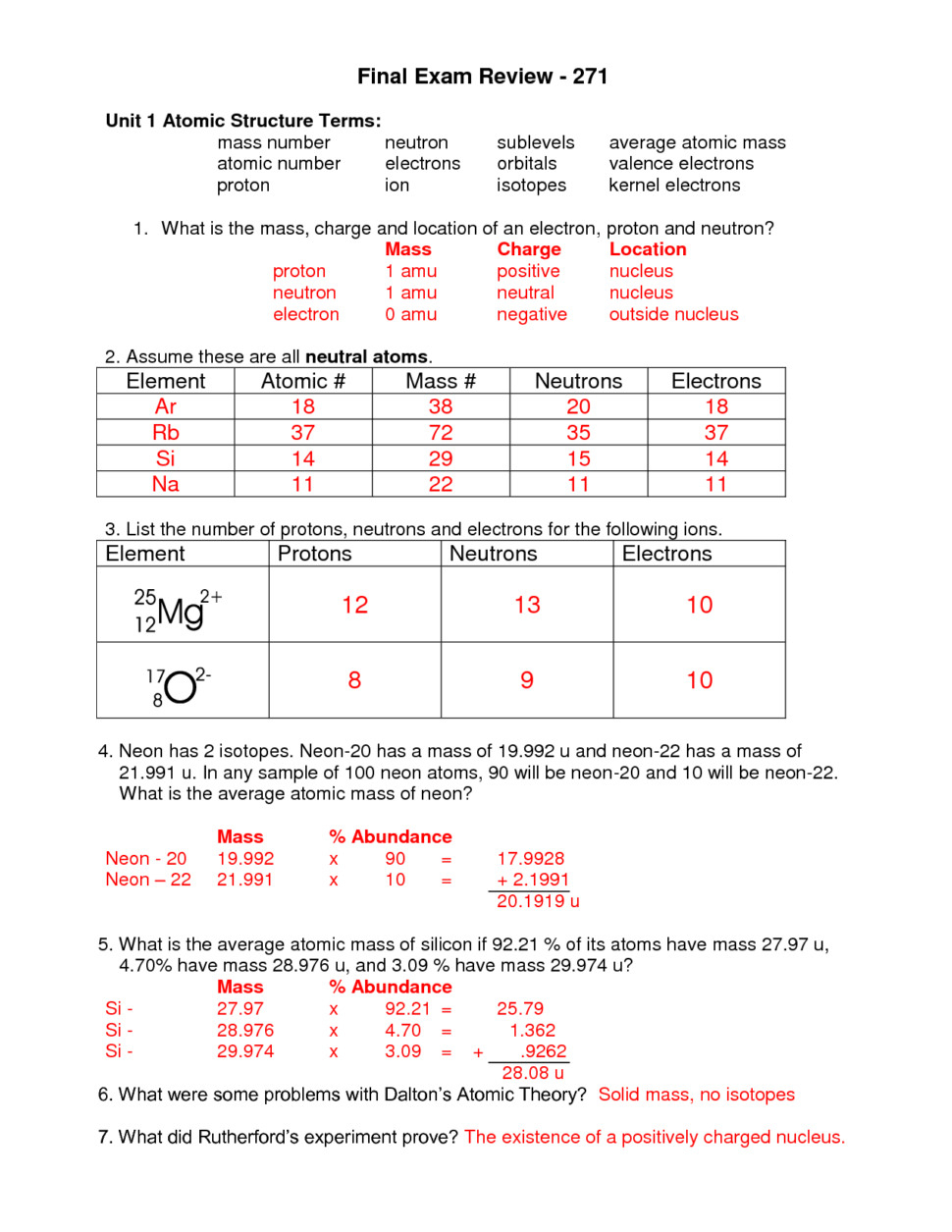
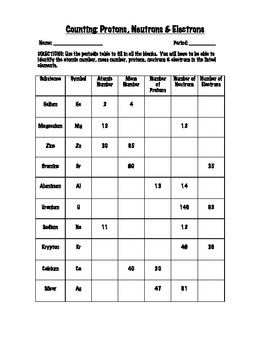
Protons, Neutrons, and Electrons Practice Worksheet Calculating the number of each particle in an atom: # Protons = Atomic Number # Electrons = Protons # Neutrons = Atomic Mass – Atomic Number OR Big # - Small # Use the periodic table to find the numbers of protons, neutrons, and electrons for atoms of the following elements. Name of Element Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas! # of protons 24 24 # of neutrons 34 39 # of electrons 24 24 Nitrogen-15 Nitrogen-20 # of protons 7 7 # of neutrons 8 13 # of electrons 7 . 7 Sulfur-23 Sulfur-25 # of protons 16 16 # of neutrons 7 18 # of electrons 16 16 Selenium-50. Selenium-55 # of protons 16 16 # of neutrons 34 39 # of electrons 16 16 Sodium-12 Sodium-20 # of protons 11 11 ...
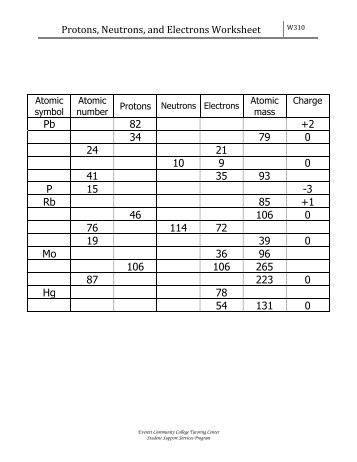
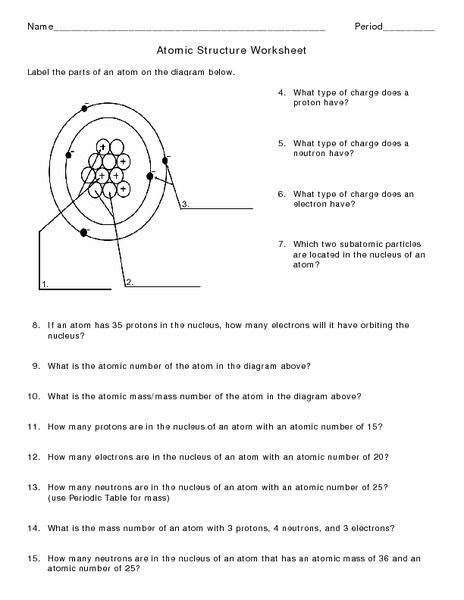
Atoms protons neutrons electrons worksheet. Protons, Neutrons, and Electrons Worksheet W310 Everett Community College Tutoring Center Student Support Services Program Atomic symbol Atomic number Protons Neutrons Electrons Atomic mass Charge Pb 82 82 125 80 207 +2 Se 34 34 45 34 79 0 Cr 24 24 28 21 52 +3 F 9 9 10 9 19 0 Nb 41 41 52 35 93 +5 P 15 15 16 18 31 -3 Rb 37 37 48 36 85 +1 Dec 10, 2021 · Protons Neutrons and Electrons Practice Worksheet Fill in the blanks in the following worksheet. Atomic symbol Atomic number Protons s Neutron Electron s. Your cells have the capability to create antioxidants with a protein called nrf2. The protons and neutrons are found in the nucleus of the atom. Most hydrogen atoms contain no neutrons, but some contain either one or two neutrons. Each atom of helium is guaranteed to contain 2 protons. Unless the atom is electrically charged, it will contain 2 electrons as well to balance the charge of the protons. Most helium atoms contain 2 neutrons, but some may contain more or less than 2. When given the protons and electrons, indicate the ion with the correct charge. Ion Protons Electrons Protons Electrons Ion Cl1- 56 54 K1+ 87 86 S2- 84 86 Sr2+ 50 46 Al3+ 32 36 P3- 55 54 Si4- 12 10 Use your periodic table to complete the table below. The first one has been done for you. Element Atomic # Mass Protons Neutrons Electrons Symbol
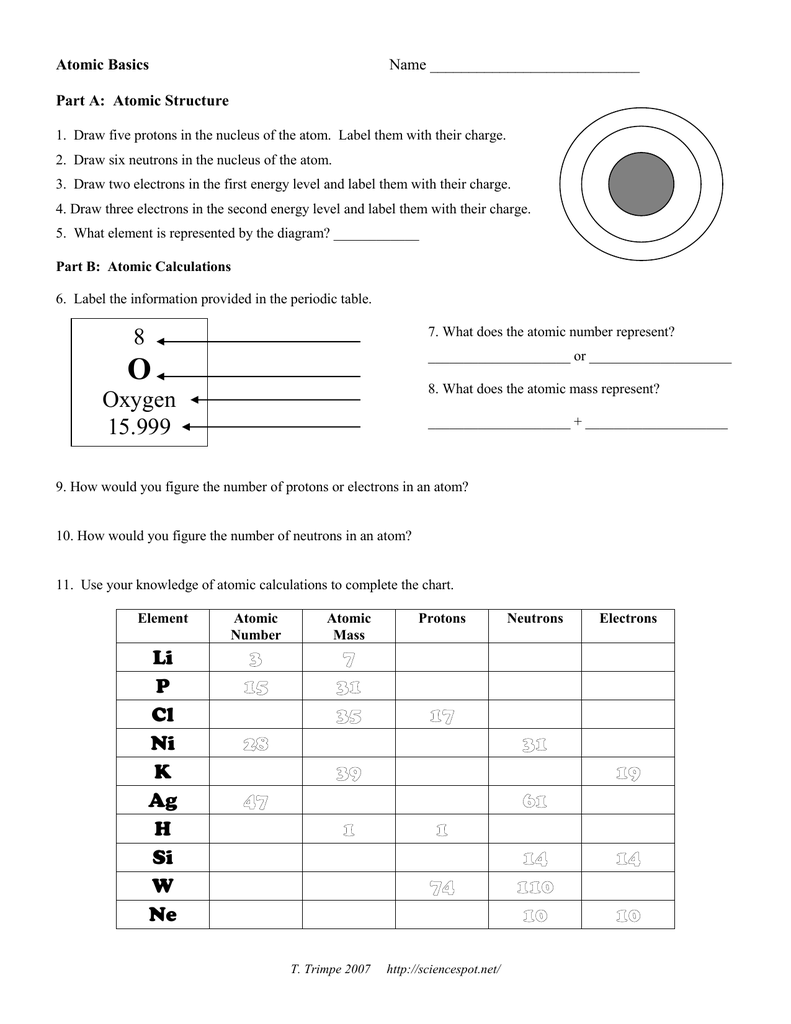
Number of Protons/Electrons: 21 Number of Neutrons: 24 Classification: Transition Metal Crystal Structure: Hexagonal Density @ 293 K: 2.989 g/cm 3 Color: silvery Atomic Structure : Number of Energy Levels: 4 First Energy Level: 2 Second Energy Level: 8 Third Energy Level: 9 Fourth Energy Level: 2 Isotopes. Isotope: Half Life: Sc-44: 3.92 hours ... Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon 6 12 6 6 6 l Hydrogen 1 1 1 0 1 H Lithium 3 7 3 4 3 Li Dec 14, 2021 · The three main ones are protons and neutrons, which are found in the nucleus or core of the atom, and electrons, which are found floating around in shells outside of the nucleus. Physicists have recently divided atoms into even smaller subatomic particles such as fermions (quarks, leptons, neutrinos, electrons) and bosons (gluons, photons ... # of protons 24 24 # of neutrons 34 39 # of electrons 24 24 Nitrogen-15 Nitrogen-20 # of protons 7 7 # of neutrons 8 13 # of electrons 7 . 7 Sulfur-23 Sulfur-25 # of protons 16 16 # of neutrons 7 18 # of electrons 16 16 Selenium-50. Selenium-55 # of protons 16 16 # of neutrons 34 39 # of electrons 16 16 Sodium-12 Sodium-20 # of protons 11 11 ...
Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas! Protons, Neutrons, and Electrons Practice Worksheet Calculating the number of each particle in an atom: # Protons = Atomic Number # Electrons = Protons # Neutrons = Atomic Mass – Atomic Number OR Big # - Small # Use the periodic table to find the numbers of protons, neutrons, and electrons for atoms of the following elements. Name of Element


























0 Response to "38 atoms protons neutrons electrons worksheet"
Post a Comment