40 gas laws worksheet with answers
Combined Gas Law Problems: 1. A gas balloon has a volume of 106.0 liters when the temperature is 45.0 ¡C and the pressure is 740.0 mm of mercury. What will its volume be a t 20.0 ¡C and 780 .0 mm of mercury pressure? ... Gas Laws Worksheet answer key Author: Lauren Peace Sep 05, 2021 · The ideal and combined gas laws worksheet answers this is likewise one of the factors by obtaining the soft documents of this the ideal and combined gas laws worksheet answers by online. Updated 4 23 2019 gas laws named after people. Combined gas law worksheet answer key is a computer program developed by researcher robert lawlor.
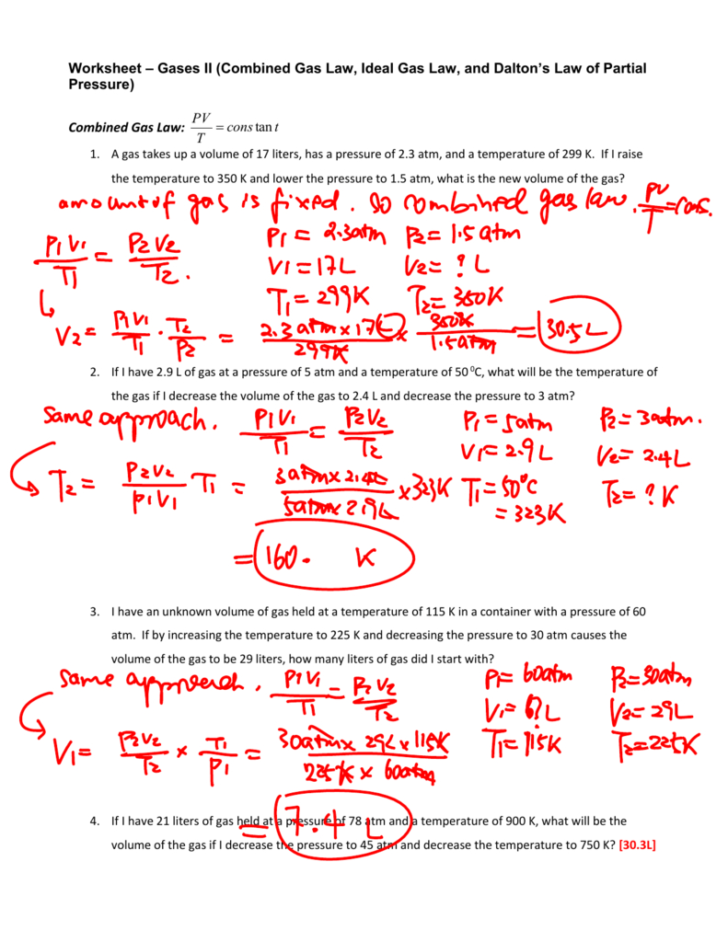
THE COMBINED GAS LAW! Use the combined gas law to solve the following problems: 1) If I initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 K, and then I raise the pressure to 14 atm and increase the temperature to 300 K, what is the new volume of the gas? 2) A gas takes up a volume of 17 liters, has a ...

Gas laws worksheet with answers
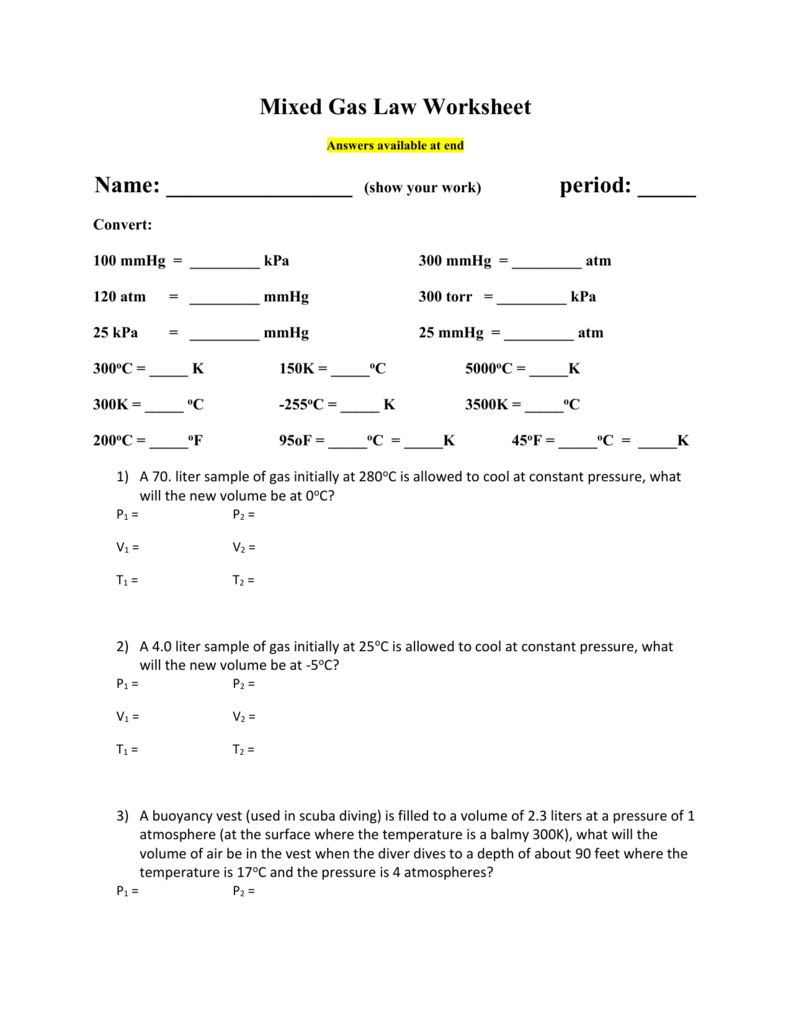
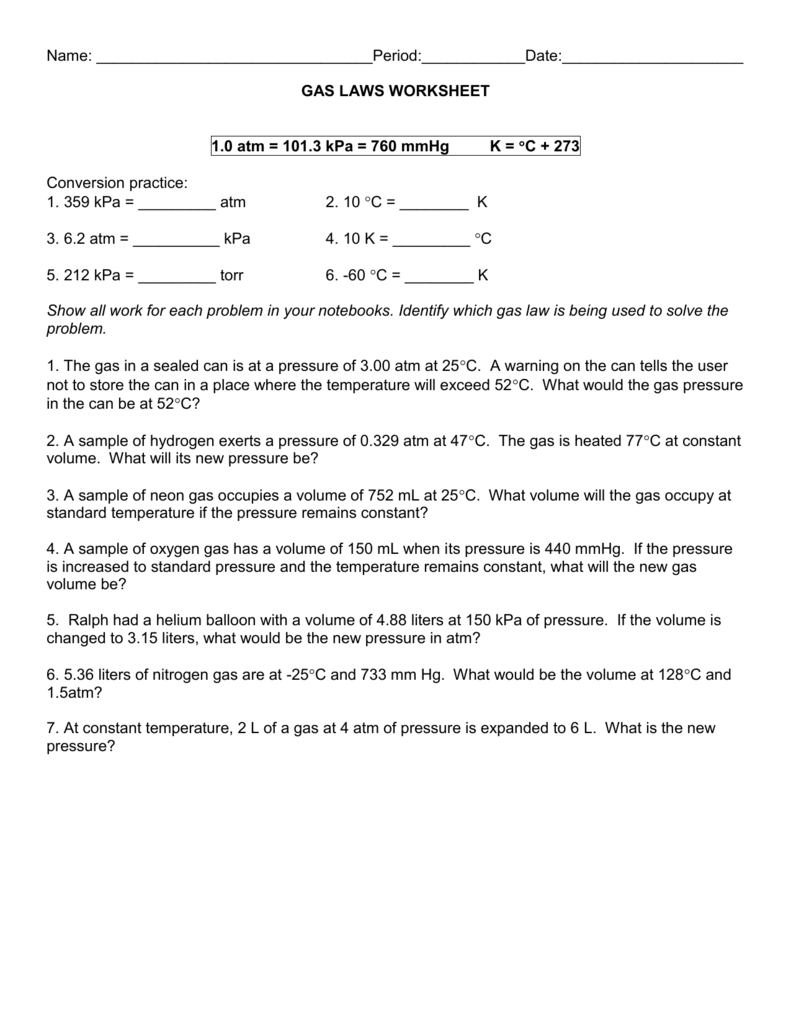
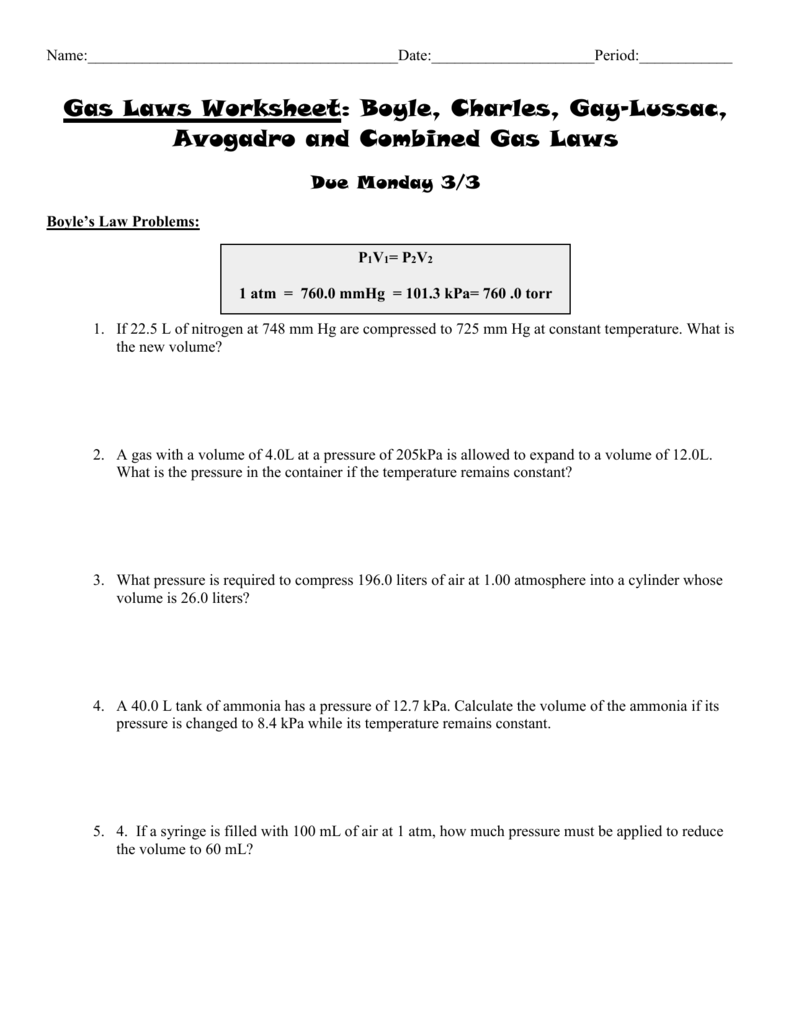
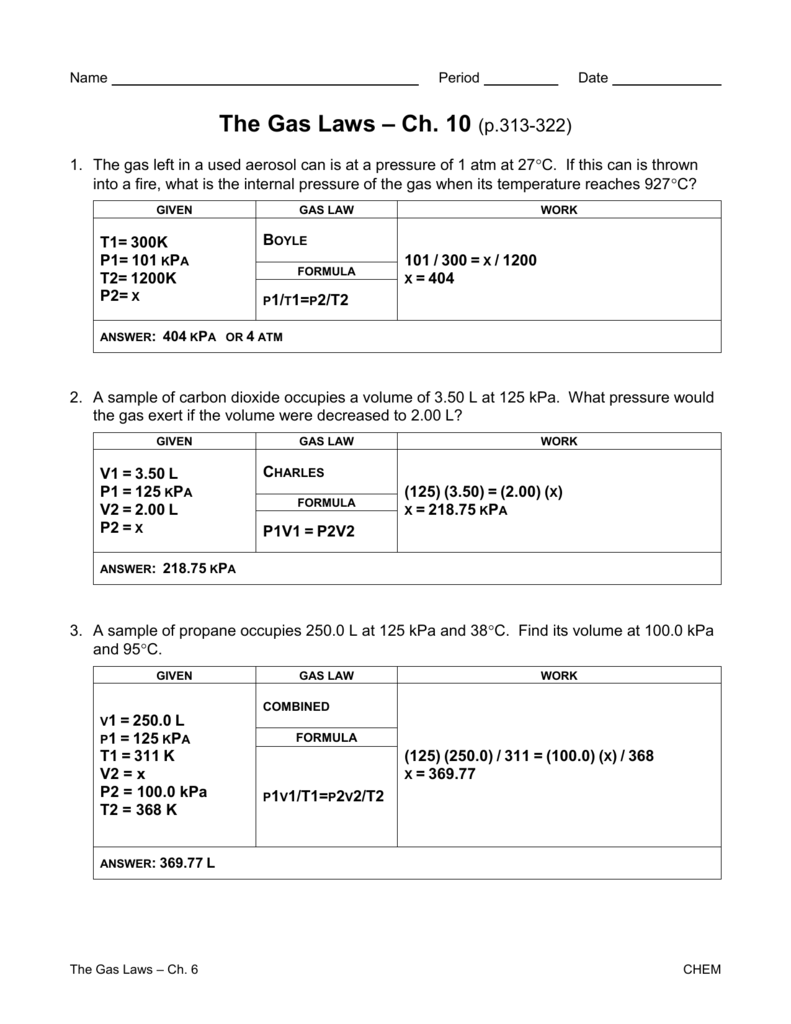
CHEMISTRY GAS LAW’S WORKSHEET 5. A sample of gas has a volume of 215 cm3 at 23.5 °C and 84.6 kPa. What volume will the gas occupy at STP? 4. 8.98 dm3 of hydrogen gas is collected at 38.8 °C. Find the volume the gas will occupy at -39.9 °C if the pressure remains constant. 3. A sample of nitrogen gas Worksheet Gas Laws Chapter 6 Boyles Law (6.3) P1V1 = P2V2 Charles Law (6.4) V1 = V2 T1 T2 Guy-Lussac’s Law (6.5) P1 = P2 T1 T2 Combined Gas Law (6.6) P1V1 = P2V2 T1 T2 Avogadros Law and STP (6.7) Standard T = 0 oC & Standard P =1 atm V1 = V2 Homework Packet: Gas Law. Boyle’s Law Problems: P1V1= P2V2. 1 atm = 760.0 mm Hg = 101.3 kPa. If 22.5 L of nitrogen at 748 mm Hg are compressed to 725 mm Hg at constant temperature. What is the new volume? A gas with a volume of 4.0L at a pressure of 205kPa is allowed to expand to a volume of 12.0L.
Gas laws worksheet with answers. Student Worksheet for Chemical Gas Laws Attempt to work the following practice problems after working through the sample problems in the videos. Answers are given on the last page(s). Relevant Equations Gas Laws Moles and Rates Boyle’s Law: P 1 V 1 = P 2 V 2 Molar Mass: Σ 𝐴 K I𝑖𝑐 𝑖 ℎ P O 𝑖 𝑐 Q What is the formula for the ideal gas law? PV = nRT. What is the value for R in the ideal gas law? 8.31 L∙kPa / K∙mol. What does R stand for in the ideal gas law? the ideal gas law constant. What does n stand for in the ideal gas law? the number of moles. Short Answer: What happens to the volume of a gas if the temperature is increased? the ... GAS LAW PROBLEMS 1. If a gas at occupies 2.60 liters at a pressure of 1.00 atm, what will be its volume at a pressure of 3.50 atm? 2. A gas occupies 900.0 mL at a temperature of 27.0 °C. What is the volume at 132.0 °C? 3. What change in volume results if 60.0 mL of gas is cooled from 33.0 °C to 5.00 °C? 4. Oct 26, 2021 · Combined gas law worksheet answers but end up in harmful downloads. A gas at 110kpa at 30 0 c fills a flexible container with an initial volume of 2 00l. If the temperature where the balloon is released is 20 0 c what will happen. If the final temperature is 30 c the final volume is 5 7 l and the final. Combined gas law worksheet 1. The ...
Gas Laws Worksheet atm = 760.0 mm Hg = 101.3 kPa= 760 .0 torr Boyle’s Law Problems: 1. If 22.5 L of nitrogen at 748 mm Hg are compressed to 725 mm Hg at constant temperature. What is the new volume? 2. A gas with a volume of 4.0L at a pressure of 205kPa is allowed to expand to a volume of 12.0L. Mixed Gas Laws Worksheet 1) How many moles of gas occupy 98 L at a pressure of 2.8 atmospheres and a temperature of 292 K? 2) If 5.0 moles of O 2 and 3.0 moles of N 2 0are placed in a 30.0 L tank at a temperature of 25 C, what will the pressure of the resulting mixture of gases be? Homework Packet: Gas Law. Boyle’s Law Problems: P1V1= P2V2. 1 atm = 760.0 mm Hg = 101.3 kPa. If 22.5 L of nitrogen at 748 mm Hg are compressed to 725 mm Hg at constant temperature. What is the new volume? A gas with a volume of 4.0L at a pressure of 205kPa is allowed to expand to a volume of 12.0L. Worksheet Gas Laws Chapter 6 Boyles Law (6.3) P1V1 = P2V2 Charles Law (6.4) V1 = V2 T1 T2 Guy-Lussac’s Law (6.5) P1 = P2 T1 T2 Combined Gas Law (6.6) P1V1 = P2V2 T1 T2 Avogadros Law and STP (6.7) Standard T = 0 oC & Standard P =1 atm V1 = V2
CHEMISTRY GAS LAW’S WORKSHEET 5. A sample of gas has a volume of 215 cm3 at 23.5 °C and 84.6 kPa. What volume will the gas occupy at STP? 4. 8.98 dm3 of hydrogen gas is collected at 38.8 °C. Find the volume the gas will occupy at -39.9 °C if the pressure remains constant. 3. A sample of nitrogen gas

























0 Response to "40 gas laws worksheet with answers"
Post a Comment