40 emission spectra and energy levels worksheet
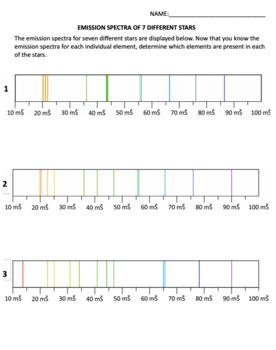
ery element has a unique atomic emission spectrum, as shown by the examples of mercury (Hg) and strontium (Sr). Classical theory was unable to explain the existence of atomic emission spectra, also known as line-emission spectra. According to classical physics, a ground state atom would be able to absorb any amount of energy rather than only 2 The emission spectrum consists of discrete lines corresponding to the differences in energy levels characteristic of and unique along the atoms of the element. Emission Spectra of 10 Elements Answer Key p5 Have students examine the. Photon of radiation that shows up bow this range line-emission spectrum. Worksheet 1 contains the spectra for 7 ...
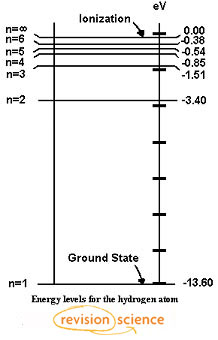
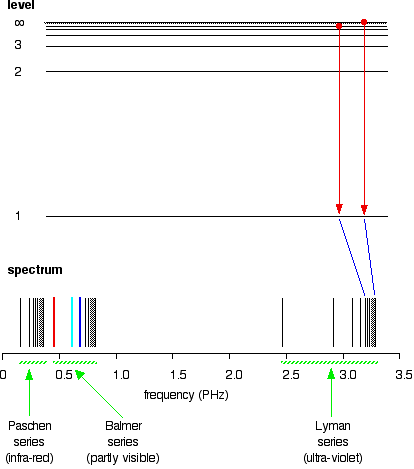
The three groups of lines in the hydrogen spectrum correspond to the transition of electrons from higher energy levels to lower energy levels. 5.3. The three groups of lines in the hydrogen spectrum correspond to the transition of electrons from higher energy levels to lower energy levels. The Lyman series corresponds to the transition to the n

Emission spectra and energy levels worksheet
Emission spectra lesson plans and worksheets from thousands of teacher reviewed resources to. The energy levels in an atom are discrete and only. Some of the worksheets displayed are atomic emission spectra energy levels and atomic spectra practice problem set 5 atomic emission spectroscopy the Exciting an electron - absorption spectra. For emission spectra of levels worksheet worksheet worksheet and emission spectra and energy levels worksheet pdf is added to perform a pdf template. If, my example, a uranium compound with. When an electron absorbs sufficient energy it moves to a higher energy level to produce an excited state. Start Practising. In this worksheet, we will practice explaining the emission of fixed colors of light by metal atoms and using line spectra in identifying elements. Q1: The atomic emission spectra of sodium and thallium are shown below. When placed into the flame of a Bunsen burner, sodium gives a vivid yellow flame.
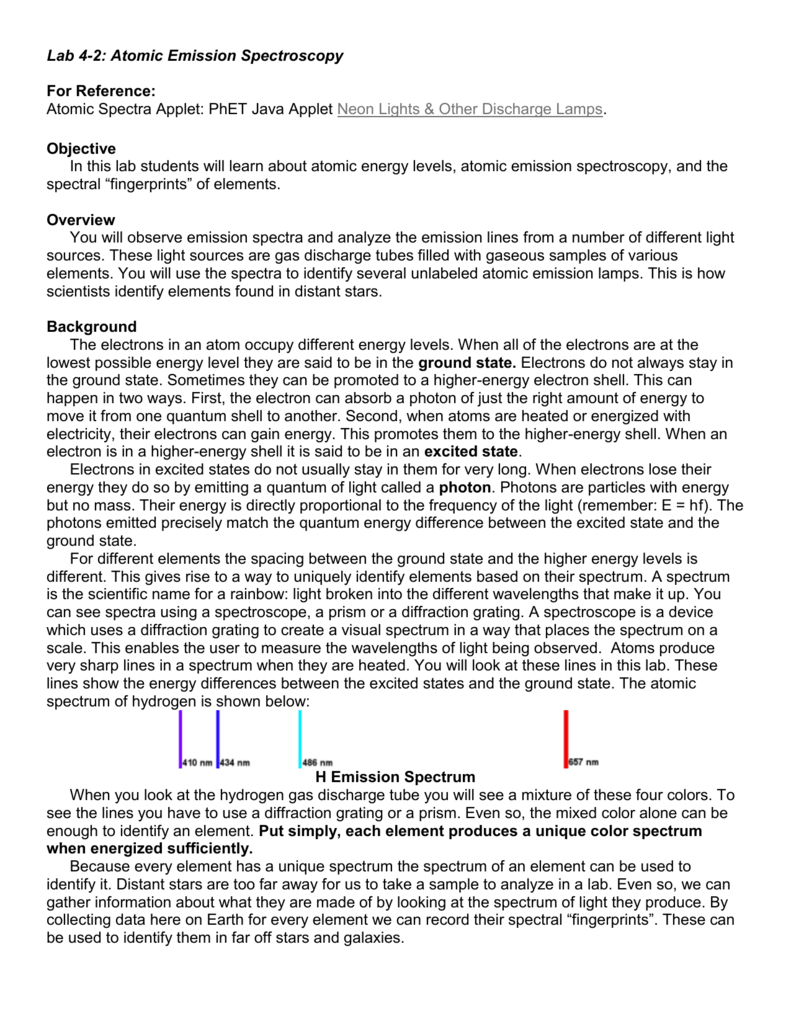
Emission spectra and energy levels worksheet. 2 On the energy level diagram show the electron transitions that would result in the series of lines that are seen in the visible region of the hydrogen emission spectrum. 3 On the energy level diagram show the electron transitions that would result in the series of lines that are seen in the infrared region of the hydrogen emission spectrum. Computer, Lab Worksheet 2. Objectives: A. Students will be able to observe the bright line spectra (emission spectra) ... unstable and eventually fall back down to their lower energy levels (ground states) releasing the energy that they had gained when they were initially excited. This energy is released in the form of light and it is Emission spectra and energy levels worksheet answers. Worksheet emission and absorption spectra answers. 5.3 atomic emission spectra worksheet answers. Supposing, we had a sample of a chemical product, but did not know what chemical was. How could we know? One way BEA would be to give a small sample of the chemical product and to burn it. energy produced by the movement of an electron from one discrete energy level to another. Thus, emission spectra are experimental proof that electrons exist at definite, distinctive energy levels in an atom. Problem: In this experiment, you will study the emission spectra of three elements: hydrogen, neon, and helium. You will
Free PDF download of HC Verma Solutions for Class 11 Physics Part-1 Chapter 9 - Centre of Mass, Linear Momentum, Collision solved by Expert Physics Teachers on Vedantu.com. All the exercise of Chapter 9 - Centre of Mass, Linear Momentum, Collision questions with Solutions to help you to revise complete Syllabus and Score More marks. Part B: The Emission Spectrum of Hydrogen - Use the Vernier system to find the hydrogen wavelengths. Recall: Part C: Calculations for the Energy Levels of Hydrogen Atom Find the energy level ε n (in kJ/mol) for each quantum number from 1 through 8 using the following equation: Example: The n=1 energy level can be calculated as follows: ε Oxybenzone is a benzophenone derivative used as a sunscreen agent. Oxybenzone absorbs UVB and UVA II rays, resulting in a photochemical excitation and absorption of energy. Upon return to ground state, the absorbed energy results in emission of longer wavelength radiation and decreased skin penetration of radiation which reduces the risk of DNA damage. Lesson Worksheet: Emission and Absorption Spectra. In this worksheet, we will practice determining the composition of a material from the features that appear in the spectrum of light coming from it. A scientist has a sample of an unknown gas. In order to identify it, she shines a continuous spectrum of white light through the gas and observes ...
An emission spectrum involves transitions of electrons from _____ to _____ energy states. An absorption spectrum involves transitions of electrons from _____ to _____ states. These transitions occur only between discrete energy levels, and thus the lines occur only at certain wavelengths and at no others. PART 1: Connec0ng electron energy levels to emission spectra. Materials: Energy level diagrams for the 10 different elements (p9-‐p18). Full spectrum “bookmark” ...18 pages Chemistry 101 8-ATOMIC EMISSION SPECTRA. Knowledge of the arrangement of electrons around the nuclei of atoms has been obtained by examining the light emitted by excited atoms.Atoms become excited when they absorb energy; they then emit energy in the form of light as they return to a less excited state.Under The maximum photon energy that will stimulate receptors in this eye is 4.97 10-19 J. 2. Blue-violet lines of the hydrogen emission spectrum are shown on page 175 of the Student Text. Recall that all of the visible photons emitted by hydrogen atoms involve electron energy-level transitions from higher levels down to a final level ofn f = 2.
And emission spectra are simply the reverse! SINCE EACH ATOM HAS A UNIQUE SET OF ENERGY LEVELS, THEIR ABSORPTION AND EMISSION SPECTRA ARE UNIQUE. THESE ALLOW US TO IDENTIFY THE ATOMS IN A GAS. Energy level transition equation. If an electron is at an excited level (E 1) ...
There is a connection between emission lines from a gas and the continuous spectrum from a solid. As you crowd atoms together (as in a solid), the allowed energy levels in one atom start to become distorted due to the influence of the electric field of neighboring atoms. Emission spectra and energy levels worksheet. There are many possible ...
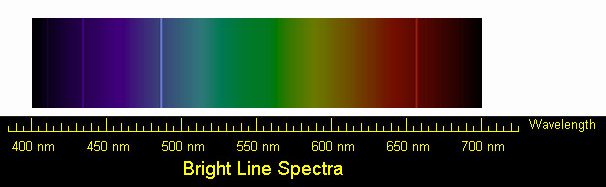
Bright-Line Spectra. Emission Spectra and. Energy Levels. Worksheet. Discussion: One convenient method of exciting atoms of an element is to pass an ...22 pages
An emission spectrum occurs when the atoms and molecules in a hot gas emit light at certain wavelengths, causing bright lines to appear in a spectrum. As with absorption spectra, the pattern of these lines is unique for each element. We can see emission spectra from comets, nebula and certain types of stars.
Emission spectra and worksheet of energy levels. Responses of the worksheet of emission and energy levels can be a valuable inspiration for those looking for an image based on specific classes you will find on this site. Thus emission spectra are experimental evidence that electrons exist in certain levels of distinctive energy in an atom.
are equipped to capture the emission spectra of dyes that emit in the 400 to 900-nm wavelength range. The resulting fluorescence and scatter are then collected and converted into electronic signals. On-board electronics convert these signals into digital data that can be acquired and recorded on the workstation.
Hollywood.com | Feel-Good Entertainment & Movie News
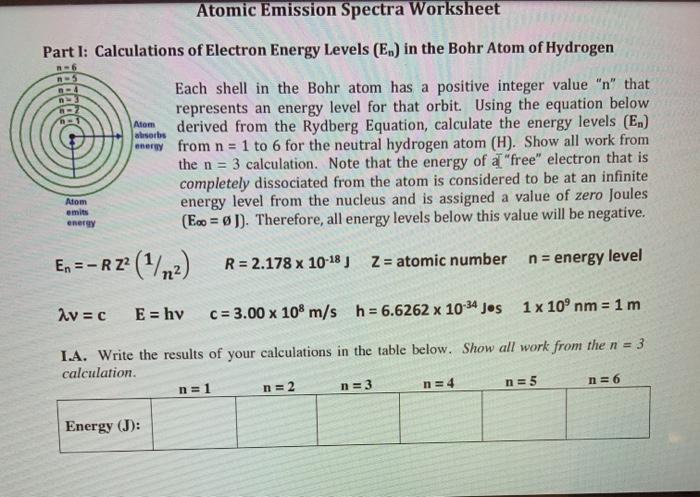
Atomic Emission Spectra Worksheet Part I: Calculations of Electron Energy Levels (E.) in the Bohr Atom of Hydrogen bebe Each shell in the Bohr atom has a positive integer value "n" that represents an energy level for that orbit.
Emission Spectra Lab Worksheet Answers emission spectra worksheet answers, 5.3 atomic emission spectra worksheet answers, interpret emission spectra worksheet answers, atomic emission spectra worksheet answers, emission spectra and energy levels worksheet answers, worksheet emission and absorption spectra answers, star emission spectrum worksheet answers
For over 40 years, mass spectra and chromatographic retention times have been accumulated in publicly available libraries under standardized conditions of 70 eV electron ionization energy, most notably in the NIST 14 Mass Spectral Library collection of the U.S. National Institute of Standards and Technology (NIST) (Babushok et al, 2007), but ...
Name: _____ Date: _____ Pd:_____ Emission Spectra and Energy Levels Worksheet Discussion: One convenient method of exciting atoms of an element is to pass an electric current through a sample of the element in the vapor phase. This is the principle behind the spectrum tubes in the demonstration.
energy level 5 to energy level 2. Refer to Models I and 2 for the following questions. a. Label the picture with "n=5 to n=2" and list the corresponding color of light emitted. b. This electron transition (absorbs e eases) ergy. c. This electron moves from a (lower/ gher) ery state to (lower igher) energy state.
Worksheet – Gynecological Conditions and Disorders of the Breast 133. Chapter 11 – MUSCULOSKELETAL 135. Worksheet – Bones (Fractures and Bone Disease) 143. Worksheet – Chronic Fatigue Syndrome 145. Worksheet – Feet 147. Worksheet – Fibromyalgia 149. Worksheet – Joints (Shoulder, Elbow, Wrist, Hip, Knee, and Ankle) 150
5.3 Atomic Emission Spectra and the Quantum Mechanical Model > 31 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. The Heisenberg ...
Name: _____ Period:_____ Page # _____ Emission Spectra and Energy Levels Worksheet Discusion: One convenient method of exciting atoms of an element is to pass an electric current through a sample of the element in the vapor phase. This is the principle behind the spectrum tubes in the demonstration. A spectrum tube contains a small sample of an element in the vapor phase.
This activity worksheet, reviews the emission spectrum of hydrogen including the electromagnetic spectrum, where students identify frequency, energy and wavelength. Labeling and describing absorption spectra and emission spectra, including a continuous spectrum, to identify the differences between t Subjects: Chemistry, Physical Science, Physics
Quantum mechanical and its electronic of the following: 2. describe the electronic structure of atoms description of the atom structure 1. enzyme action in terms of main energy levels, sublevels, STEM_GC11ES-IIa-b-53 2.
wavelength of 459 nm. What is the energy content, in joules, of this photon? (-34 8)( )-19-7-19 6.33 x 10 J s 3.00 x 10 m s = = 4.33 x 10 J 4.59 x 10 m = 4.33 x 10 J E E ⋅ 3. Emission spectrums are produced from the release of light by electrons in varying energy levels. The following is an emission spectrum of hydrogen gas. Calculate the
Name: _____ Period:_____ Page # _____ Emission Spectra and Energy Levels Worksheet Discusion: One convenient method of exciting atoms of an element is to pass an electric current through a sample of the element in the vapor phase. This is the principle behind the spectrum tubes in the demonstration.
Worksheet. 1. Which of the following best describes the reason that line emission spectra contain lines? The energy levels in an atom are discrete and only certain emission wavelengths are ...
Major Electron Level Notation Worksheet (DOCX 24 KB) Major Energy Levels (M. E. L.) of electrons Worksheet (DOC 24 KB) Practice with Major Energy Level Configurations Worksheet (DOCX 16 KB) Shell Diagrams of Electrons Worksheet (DOC 37 KB) Spectrum, Electron & Energy Levels Worksheet (DOCX 13 KB) Video - The Wave Mechanical Model Worksheet ...
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds composed of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances.. In the scope of its subject, chemistry occupies an …
Start Practising. In this worksheet, we will practice explaining the emission of fixed colors of light by metal atoms and using line spectra in identifying elements. Q1: The atomic emission spectra of sodium and thallium are shown below. When placed into the flame of a Bunsen burner, sodium gives a vivid yellow flame.
For emission spectra of levels worksheet worksheet worksheet and emission spectra and energy levels worksheet pdf is added to perform a pdf template. If, my example, a uranium compound with. When an electron absorbs sufficient energy it moves to a higher energy level to produce an excited state.
Emission spectra lesson plans and worksheets from thousands of teacher reviewed resources to. The energy levels in an atom are discrete and only. Some of the worksheets displayed are atomic emission spectra energy levels and atomic spectra practice problem set 5 atomic emission spectroscopy the Exciting an electron - absorption spectra.



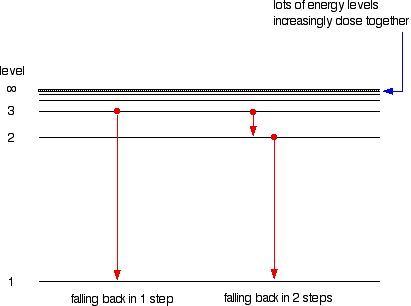


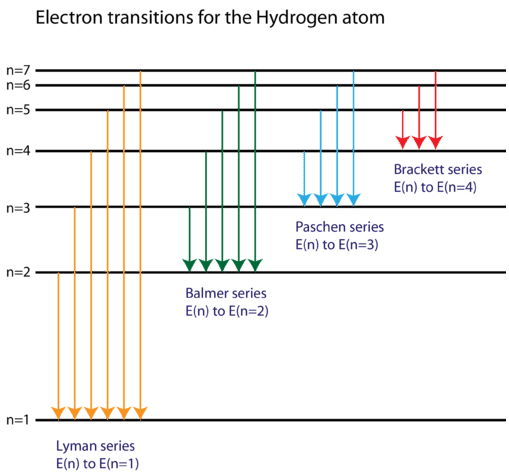

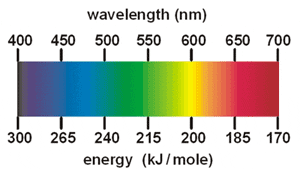

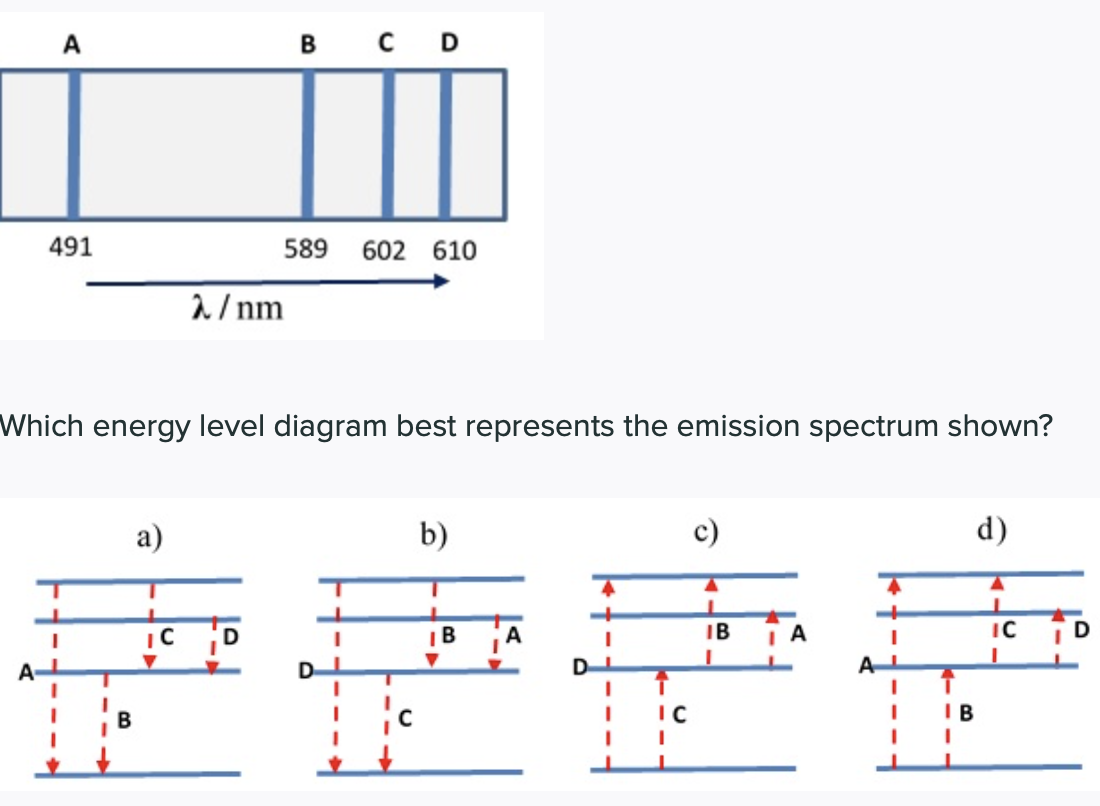















0 Response to "40 emission spectra and energy levels worksheet"
Post a Comment