40 specific heat capacity worksheet answers
PDF sch4u-specific heat and heat capacity worksheet with answers Specific Heat and Heat Capacity Worksheet 1 The temperature of 335 g of water changed from 24.5oC to 26.4oC. How much heat did this sample absorb? c for water = 4.18 J/goC (ans. 2.66 kJ) 2. How much heat in kilojoules has to be removed from 225g of water to lower its temperature from 25.0oC to 10.0oC? (ans. -14.1 kJ) 3. DOC Specific Heat Worksheet - Socorro Independent School District 1. A 15.75-g piece of iron absorbs 1086.75 joules of heat energy, and its temperature changes from 25°C to 175°C. Calculate the specific heat capacity of iron. 2. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°C to 55°C, if the specific heat of aluminum is 0.90 J/g°C? 3.
PDF Worksheet- Introduction to Specific Heat Capacities Water has the highest specific heat capacity and metal has the lowest. 6. Here are the heat capacities of the four substances: 0.10 cal/g °c, 0.25 cal/g °c, 1.0 cal/g °c, & 0.2 cal/g °c. Match & then labeleach substance with its specific heat capacity on the graph. See graph above. 7.

Specific heat capacity worksheet answers
PDF North St. Paul-Maplewood Oakdale / Overview Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 6.75x104 joules of heat, and its temperature changes from 320C to 57 oc cp. û5Dc 37500CP 100.0 of 40C water is heated until its temperature is 370C. If the specific heat of water is 4.18 J/ábc, calculate the amount of heat needed to cause this rise in temperature. PDF Worksheet Introduction To Specific Heat Capacities Answers Specific Heat Problems Worksheet Answers Specific Heat Worksheet. Specific Heat. DIRECTIONS: Use q = (m)(ΔT)(Cp) to solve the following problems. Show all work and units. A 15.75-g piece of iron absorbs 1086.75 joules of heat energy, and its temperature changes from 25°C to 175°C. Calculate the specific heat capacity of iron. Specific Heat ... PDF Specific Heat and Heat Capacity Worksheet Specific Heat and Heat Capacity Worksheet DIRECTIONS: Use q = (m)(Cp))(ΔT) to solve the following problems. Show all work and units. Ex: How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°C to 55°C, if the specific heat of aluminum is 0.90 J/g°C? 1.
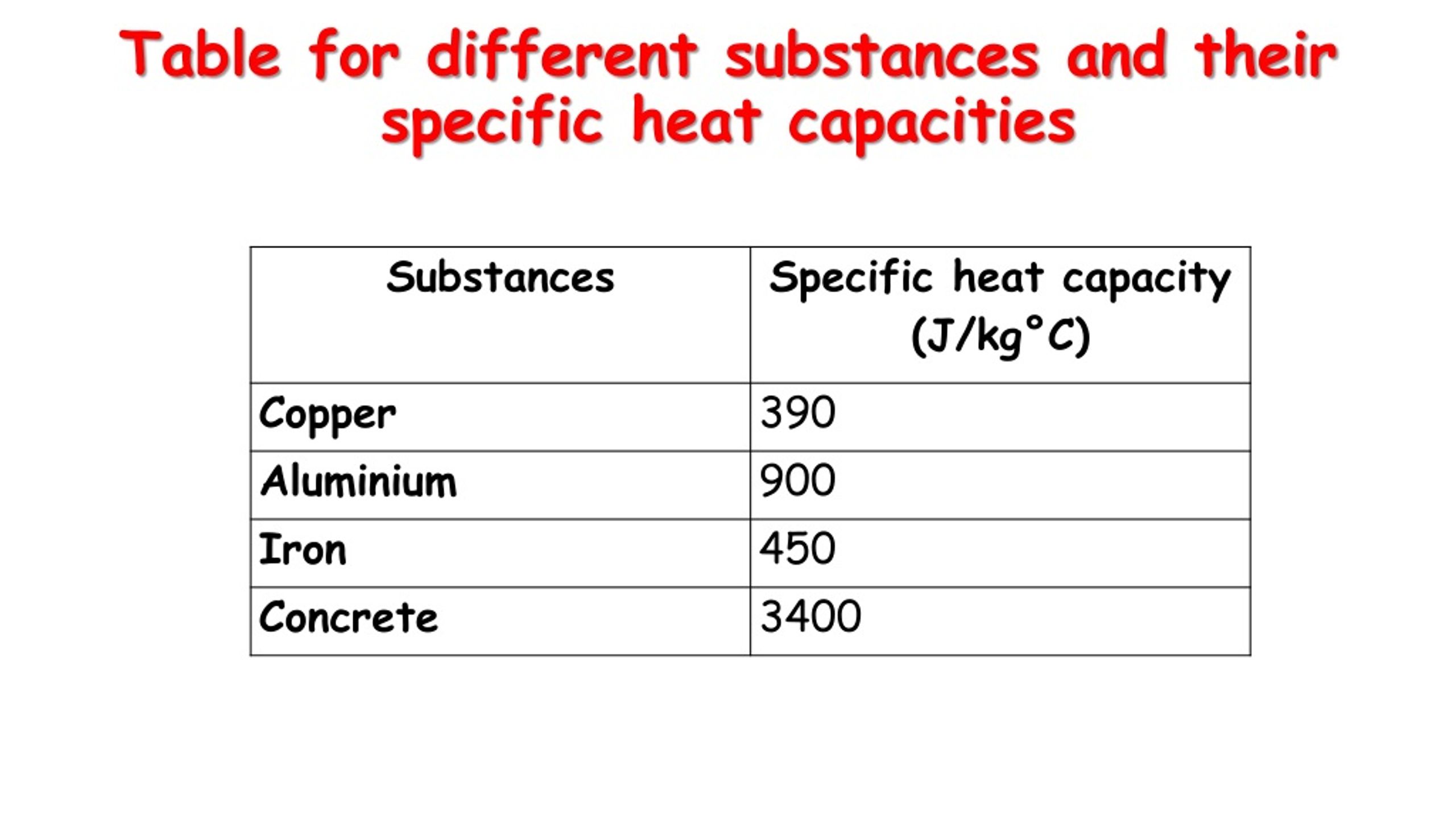
Specific heat capacity worksheet answers. Specific heat capacity worksheet - Liveworksheets.com ID: 1338126 Language: English School subject: Physics Grade/level: higher Age: 7+ Main content: Heat Other contents: Calculating specific heat capacity Add to my workbooks (6) Download file pdf Embed in my website or blog Add to Google Classroom Specific Heat Capacity 1 - Worksheet | Teaching Resources pdf, 1.48 MB This worksheet is designed for GCSE Physics students. It includes a series of questions of increasing challenge, with answers and extra supporting videos available at the link on the bottom of each page or via the QR code. Full written answers and a video explanation for this worksheet is also available. PDF Worksheet 6.1 Specific heat capacity - St Leonard's College The specific heat capacities of some common substances are shown in the table below. Substance State at 25ºC, 101 kPa Specific heat capacity (J g-1ºC-1 ) Aluminium Solid 0.90 Nickel Solid 0.44 Copper Solid 0.39 Water Liquid 4.18 Ethanol Liquid 2.46 Propanone (acetone) Liquid 2.13 No. Question Answer PDF Specific Heat Wksht20130116145212867 1. A 15.75-g piece of iron sorbs 1086.75 joules of heat energy, and its temperature changes from 25 0 1750C. Calculate the specific heat capacity of iron. = 'C ' Q 5) 2. How many joules of heat are neeaea Fo ratse the temperature of 10.0 g of aluminum from 220C to 550C, if the specific heat of aluminum is 0.90 J/g0C? 3.
plaza.ufl.edu › ctoyota › worksheet 17cgtCalculating Heat - University of Florida Worksheet 17 1 Worksheet #17 Calculating Heat 1. How much heat is needed to bring 12.0 g of water from 28.3 °C to 43.87 °C, if the specific heat capacity of water is 4.184 J/(g•°C)? 2. How much heat is released when 143 g of ice is cooled from 14 °C to – 75 °C, if the specific heat capacity of ice is 2.087 J/(g•°C). 3. Solved Specific Heat and Heat Capacity Worksheet ... Chemistry questions and answers. Specific Heat and Heat Capacity Worksheet DIRECTIONS: Use q = (m) (Cp) (AT) to solve the following problems. Show all work and units q = Heat Transfer (cal) m = mass (g) Cp = specific heat capacity (cal/g °C) Cp of Water = lcal/g °C 1. The temperature of 245 g of water changed from 20.5°C to 26.4°C. PDF Worksheet- Calculations involving Specific Heat Worksheet- Calculations involving Specific Heat 1. For q= m c Δ T : identify each variables by name & the units associated with it. q = amount of heat (J) m = mass (grams) c = specific heat (J/g°C) ΔT = change in temperature (°C) 2. Heat is not the same as temperature, yet they are related. Explain how they differ from each other. PDF Calculating Specific Heat Capacity Worksheet With Answers Calculating Specific Heat Capacity Worksheet With Answers Author: passport.dio.cuhk.edu.hk-2022-05-03-00-06-17 Subject: Calculating Specific Heat Capacity Worksheet With Answers Keywords: calculating,specific,heat,capacity,worksheet,with,answers Created Date: 5/3/2022 12:06:17 AM
PDF Name: Per: Worksheet- Introduction to Specific Heat Capacities Name:_____ Per:_____ Worksheet- Introduction to Specific Heat Capacities Heating substances in the sun: The following table shows the temperature after 10.0 g of 4 different substances have been in direct sunlight for up to 60 minutes. Honors Chemistry Worksheet - Specific Heat What is the specific heat of this object? (Ans. 0.48 cal/g oC or 2.0 J/g oC) 3. 1,200 cal of heat energy is added to a liquid with a specific heat of 0.57 cal/g°C. If the temperature increases from 20.°C to 33°C, what is the mass of the liquid? (Ans. 190 g) 4. A piece of food is burned in a calorimeter that contains 200.0 g of water. Specific Heat Capacity | Teaching Resources zkhan175. 3 years ago. report. 5. Excellent worksheet that helped me practice the Specific Heat Capacity equation in preparation for my mock exam. Empty reply does not make any sense for the end user. Submit reply. Cancel. Report this resource to let us know if it violates our terms and conditions. PDF Specific Heat Calculations Worksheet Key Created Date: 4/28/2016 8:10:49 AM
PDF Specific Heat Calculations Worksheet With Answers Specific Heat Worksheet. Specific Heat. DIRECTIONS: Use q = (m)(ΔT)(Cp) to solve the following problems. Show all work and units. A 15.75-g piece of iron absorbs 1086.75 joules of heat energy, and its temperature changes from 25°C to 175°C. Calculate the specific heat capacity of iron. Specific Heat Worksheet
Specific Heat Capacity Required Practical Worksheet for AQA GCSE Physics by jwansell - Teaching ...
AUSTIN_WASHINGTON_-_Copy_of_specific_heat_and_heat ... Specific Heat and Heat Capacity Worksheet q = m x Cp x ∆T q = heat energy, m = mass T = temperature ∆T = (T final - T initial) Show all work and proper units. WRITE THE FORMULA FOR EACH PROBLEM FOR FULL CREDIT. 1 The temperature of 335 g of water changed from 24.5 o C to 26.4 o C.
DOC Calculating Specific Heat Worksheet - Council Rock School ... Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes from 32°C to 57°C. 4. 100.0 g of 4.0°C water is heated until its temperature is 37°C. If the specific heat of water is 4.18 J/g°C, calculate the amount of heat energy needed to cause this rise in ...
ANSWER KEY - west-windsor-plainsboro.k12.nj.us Hall of fame question- depends on your answers for 1-5: A brilliant chemistry student harnesses all of the energy released from problems 1, 2, and 4 to heat up an unknown sample with 1000 times the mass of water in problem 3. She raises the temperature from 50 C to the same final temperature as problem 5.
PDF Specific Heat Capacity - Step Up In Education 5.00 0C and has a specific heat capacity of 385 Jkg-1K . Calculate the change in temperature of the aluminium. 10. Ethylene glycol has half the specific heat capacity of water. A sample of ethylene glycol was heated on an element that was set to 80% the power that was used to heat a 1.00 L sample of water. What is the mass
Specific Heat Capacity - Worksheet (Key) - Specific Heat ... Specific Heat Capacity specific heat capacity tl fi nc au296r?!)j 7t2 tet pc6f kl ti (xt, how much heat is up 36 kg of hydrogen gas from 12.0 to
DOC Specific Heat Worksheet - Winston-Salem/Forsyth County Schools (Cp of H2O = 4.184 J/g °C) 11. How much energy is required to heat 120.0 g of water from 2.0 °C to 24.0 °C? (Cp of H2O = 4.184 J/g °C) 12. How much heat (in J) is given out when 85.0 g of lead cools from 200.0 °C to 10.0 °C? (Cp of Pb = 0.129 J/g °C) 13.
study.com › learn › lessonLatent Heat Vaporization Formula & Examples | What is Latent ... Dec 20, 2021 · Latent heat is the heat needed to cause a phase change to a specific amount of matter at a fixed temperature. It is the amount of heat that is absorbed or released divided by the amount of ...
study.com › skill › learnHow to Calculate Final Temperature of an Object after Heat ... Step 2: Plug in the mass, specific heat, and amount of heat from the previous step into the rearranged equation for specific heat: {eq}\Delta T=\frac{Q}{m\cdot c} {/eq}.
PDF Specific Heat Worksheet Answers Key Acces PDF Specific Heat Worksheet Answers Key most operational sellers here will categorically be in the middle of the best options to review. 20V Specific Heat worksheet 20T Specific Heat worksheet Calorimetry Examples: How to Find Heat and Specific Heat Capacity Specific Heat Capacity 2 - GCSE Physics Worksheet Answers EXPLAINED How to
Specific Heat Worksheet Answer Key - bossinspire The specific heat capacity of iron 0 449 j g0c and water 4 18 j g0c. Answers are provided at the end of the worksheet without units. Specific heat worksheet name (in ink): Specific heat capacity tl fi nc au296r j 7t2 tet pc6f kl ti xt how much heat is up 36 kg of hydrogen gas from 12 0 to. Chemistry specific heat worksheet answer key december 6 ...
And Heat Capacity Worksheet Heat Answers Specific [EGYMOU] Search: Specific Heat And Heat Capacity Worksheet Answers
PDF Specific Heat Capacity Worksheet - HYDESCIENCE Specific Heat Capacity Worksheet 1. Explain what the specific heat of an object means/tells us? 2. How is knowing the specific heat of an object useful? The table to the right list the specific heat (Heat Capacity) of four common materials Aluminum, Beryllium, Gold, and Graphite. Use this table to answer questions 3 - 7 3.
Specific Heat Capacity - Worksheet (Key) | PDF ... A 15.75-9 piece of iron absorbs L}86.75joules of heat energy, and its temperature changes from 25"C to 175"C. Calculate the specific heat capacity of iron. h= lf ,7f gz o,otSZfb Q- ^, A T cx 4/o J/4'c









0 Response to "40 specific heat capacity worksheet answers"
Post a Comment