44 chemistry specific heat worksheet answers
specific heat problems answer key.pdf - ISD 622 Specific Heat Worksheet. Rey. Cp = q/mAT, where q = heat energy, m = mass, and T = temperature. 1. A 15.75-g piece of iron absorbs 1086.75 joules of heat ...1 page Heat and Heat Calculations Name___________________ ... Heat is the total kinetic energy that flows because of a difference in temperature and does depend on the amount of matter. Kinetic Energy cannot specific heat ...3 pages
PDF Specific Heat Capacity Questions and Answers - chemistry! Calculate the specific heat capacity of mercury. 7. What is the specific heat capacity of silver metal if 55.00 g of the metal absorbs 47.3 calories of heat ...5 pages
Chemistry specific heat worksheet answers
Specific Heat Chemistry Check your answer with a specific heat table. 9. 100.0 mL of 4.0°C water is heated until its temperature is 37°C. If the specific heat of water is 4.18 J ...4 pages Specific Heat Calculations Worksheet - Chemistry - LPS Specific Heat Calculations Worksheet. Chemistry. 2 points. Here is a chart of specific heat capacities for your use: Specific Heat. Capacity (J/g.°C).4 pages Worksheet- Calculations involving Specific Heat Explain how they differ from each other. Heat is a combination of kinetic energy (measured by temperature) and potential energy. a. Perform calculations using: ...2 pages
Chemistry specific heat worksheet answers. Specific Heat Worksheet q=(mass)(Csp)(AT) Specific Heat Worksheet ... What is the specific heat of a substance that absorbs 2.5 x 10³ joules of heat when ... 0°C? Explain your answer quantitatively.4 pages Specific Heat Worksheet Show all work and proper units. Answers are provided at the end of the worksheet without units. 1. A 15.75-g piece of iron absorbs 1086.75 joules of heat energy ...2 pages Specific Heat Worksheet 100.0 mL of 4.0°C water is heated until its temperature is 37°C. If the specific heat of water is 4.18 J/g°C, calculate the amount of heat energy needed to ... Worksheet- Calculations involving Specific Heat Explain how they differ from each other. Heat is a combination of kinetic energy (measured by temperature) and potential energy. a. Perform calculations using: ...2 pages
Specific Heat Calculations Worksheet - Chemistry - LPS Specific Heat Calculations Worksheet. Chemistry. 2 points. Here is a chart of specific heat capacities for your use: Specific Heat. Capacity (J/g.°C).4 pages Specific Heat Chemistry Check your answer with a specific heat table. 9. 100.0 mL of 4.0°C water is heated until its temperature is 37°C. If the specific heat of water is 4.18 J ...4 pages










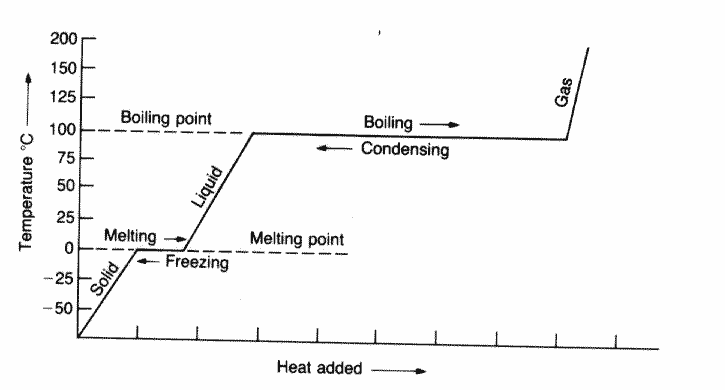











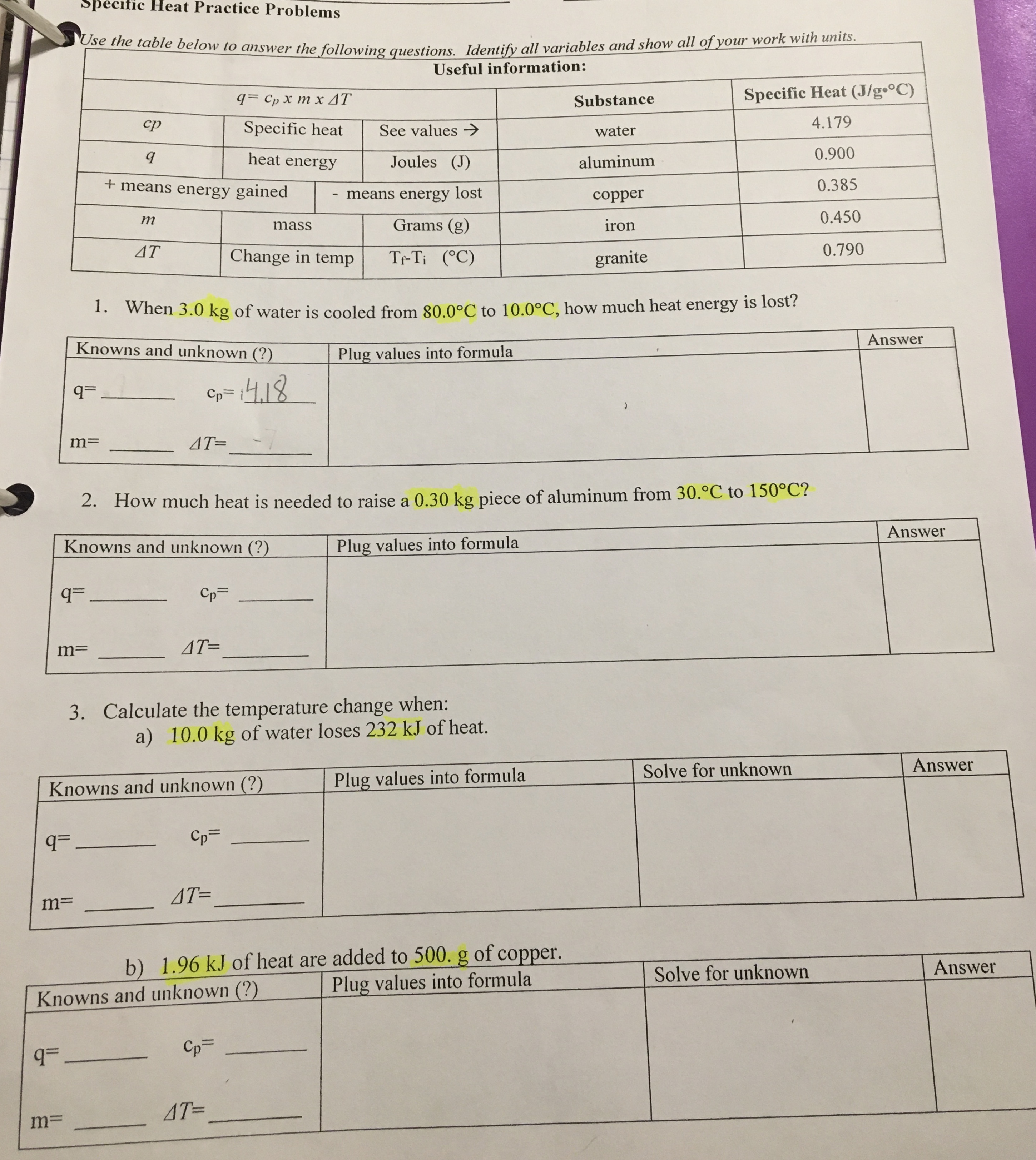







0 Response to "44 chemistry specific heat worksheet answers"
Post a Comment