38 atoms and subatomic particles worksheet
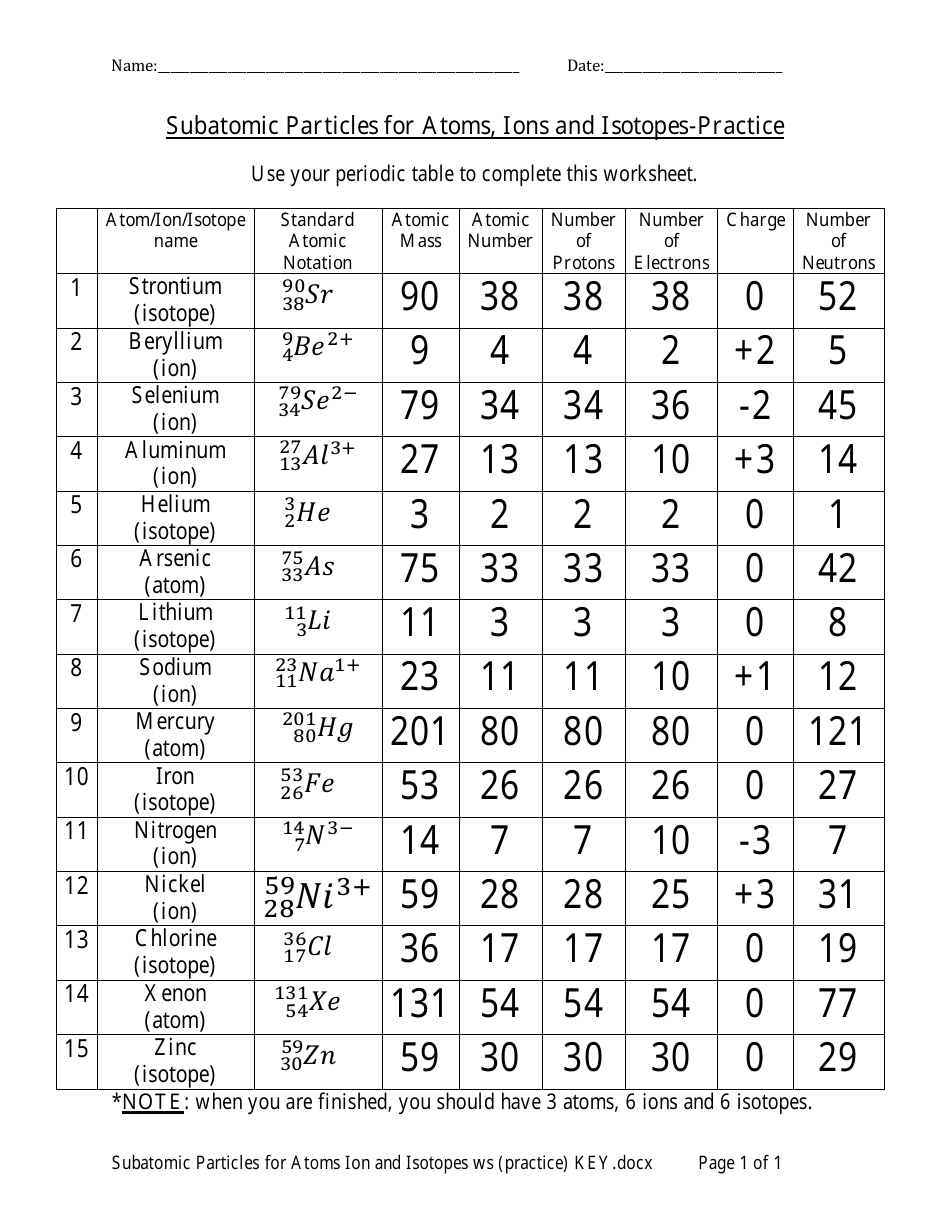
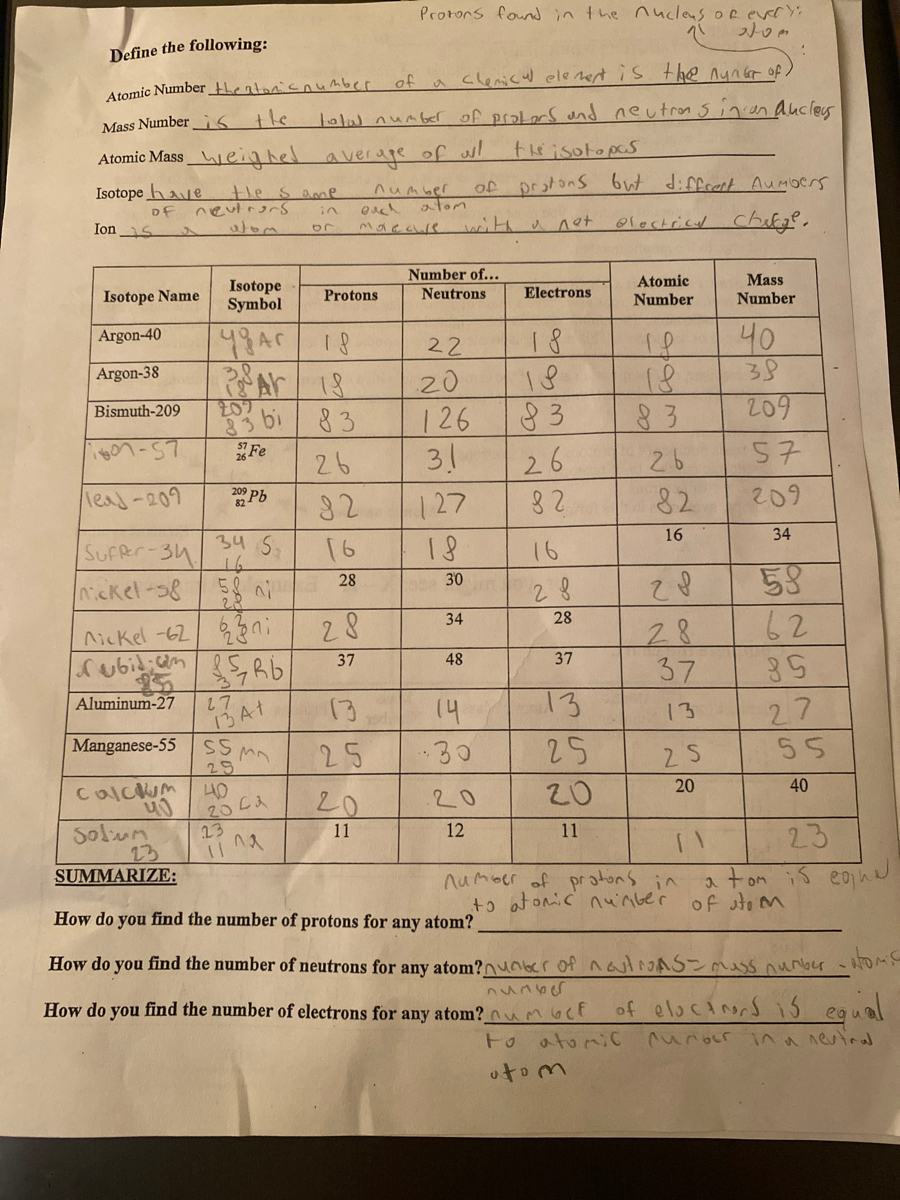
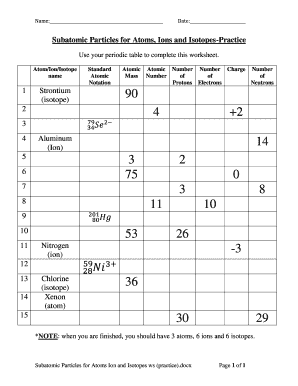
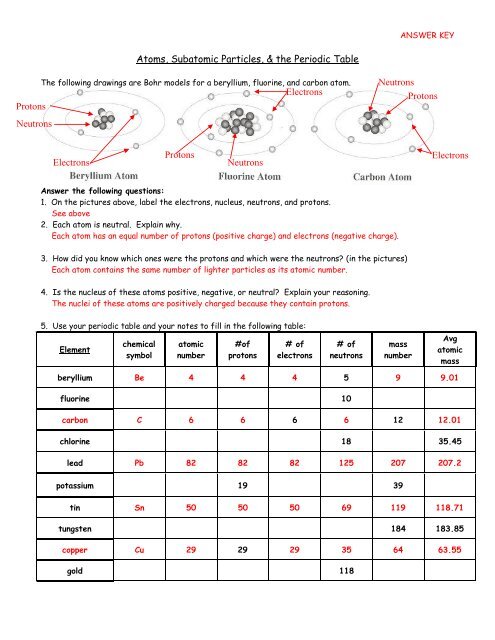
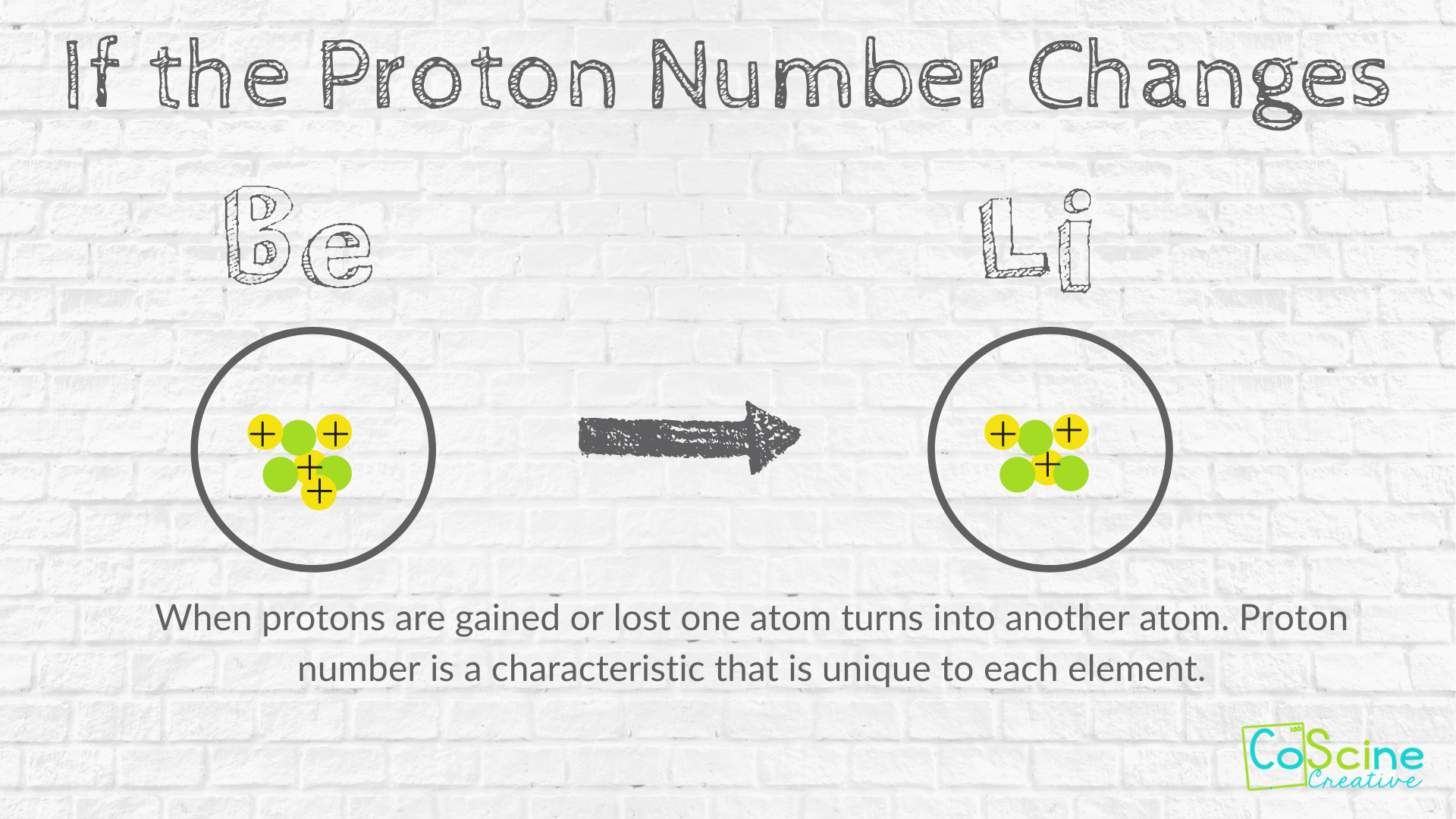
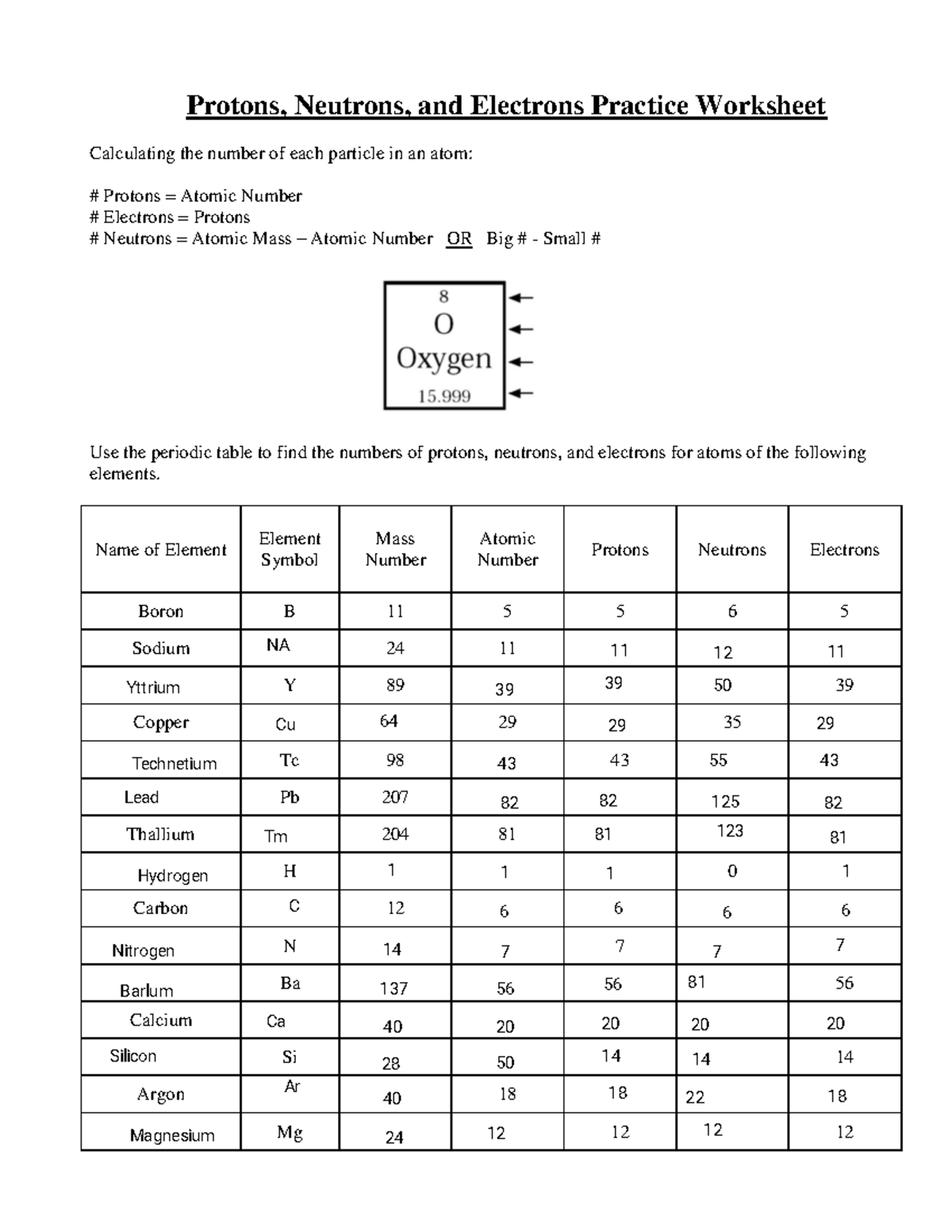
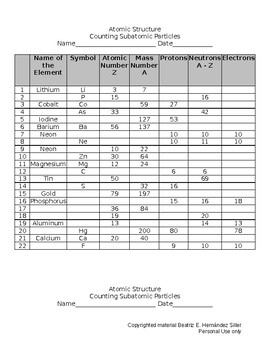
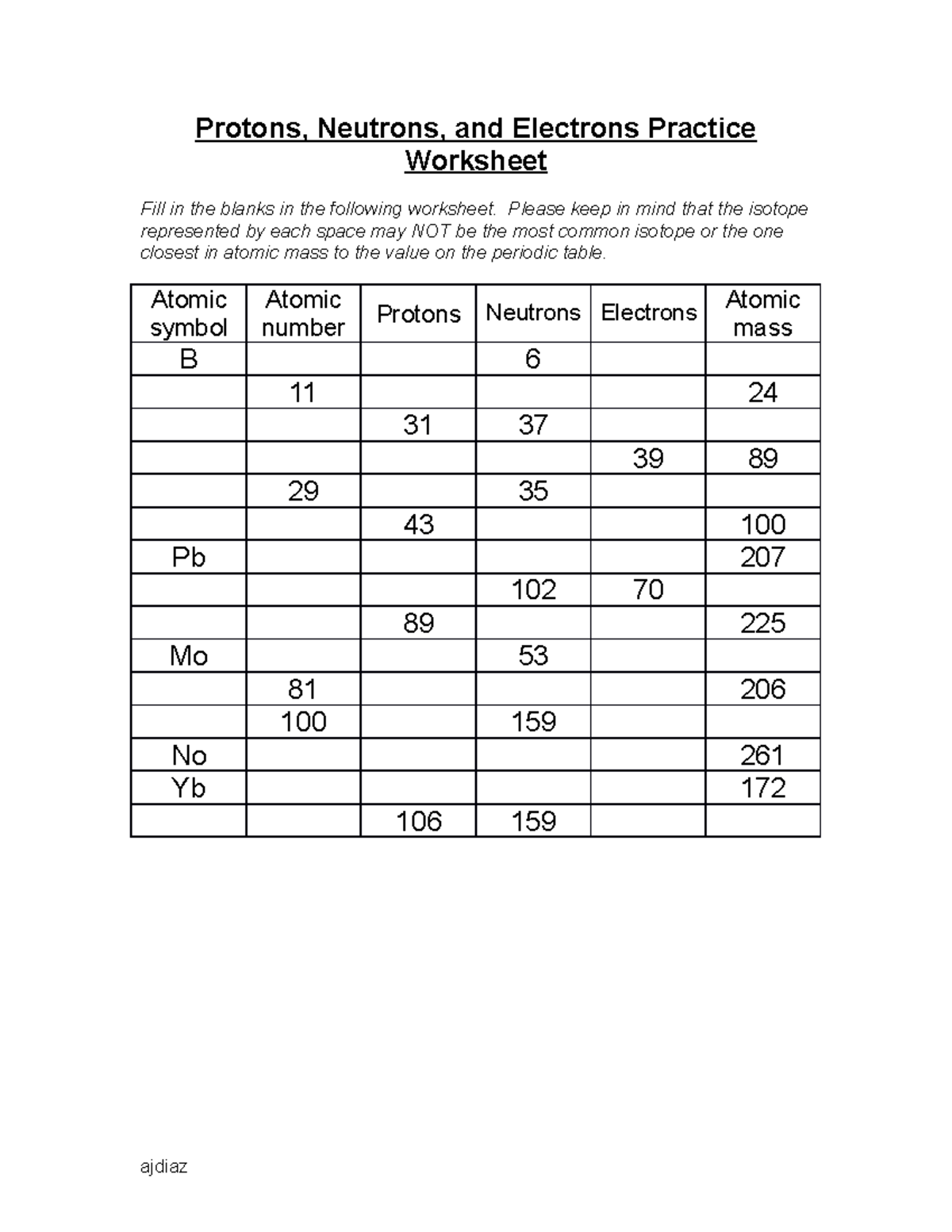
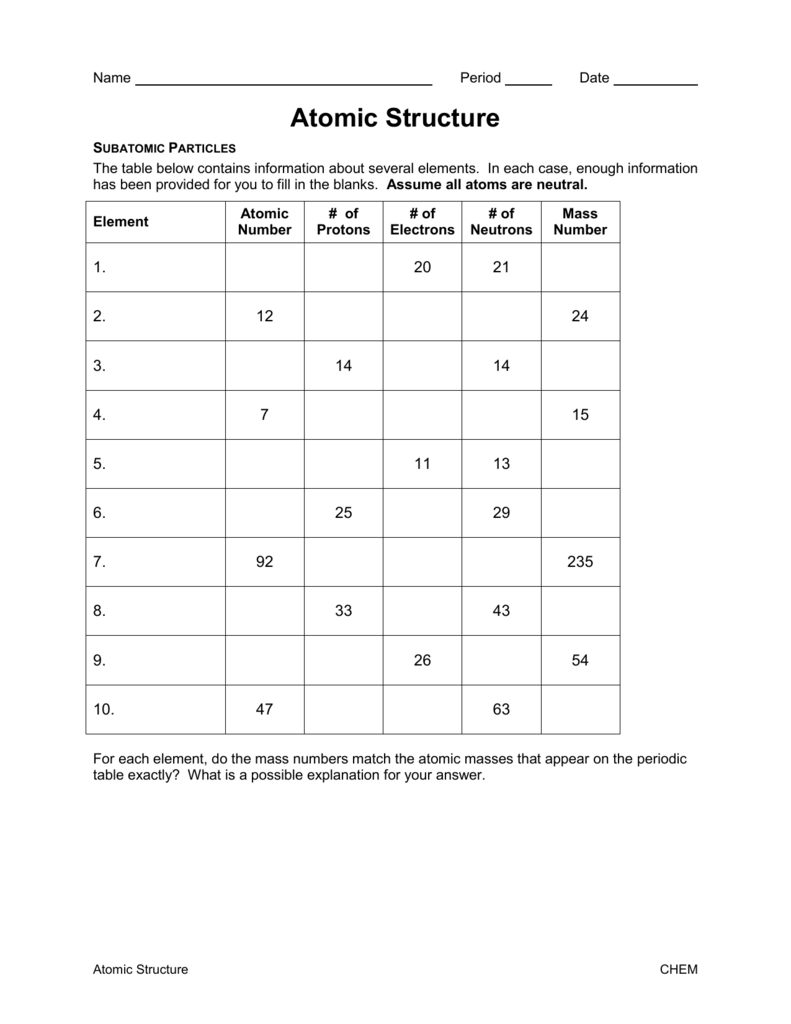
SUBATOMIC PARTICLES and ISOTOPES WORKSHEET SUBATOMIC PARTICLES and ISOTOPES WORKSHEET Complete the following table using the information discussed in class and your Periodic Table. All atoms are neutral. Element Name Atomic Number Mass Number Number of protons Number of neutrons Number of electrons Isotopic notation oxygen 8 17 8 9 8 17 8O phosphorous 15 31 15 16 15 31 15 P BrainPOP Moved Permanently. The document has moved here. Atoms - BrainPOP
Gumdrop Atoms - Activity - TeachEngineering Web25/02/2020 · Well, these tiny subatomic particles are constantly moving or vibrating and we cannot even tell. For example, if you look at a molecule of water, it is made up of two hydrogen atoms and one oxygen atom. The electrons in each of these atoms that make up the water molecule are moving all around in their electron shells, or clouds. The …

Atoms and subatomic particles worksheet
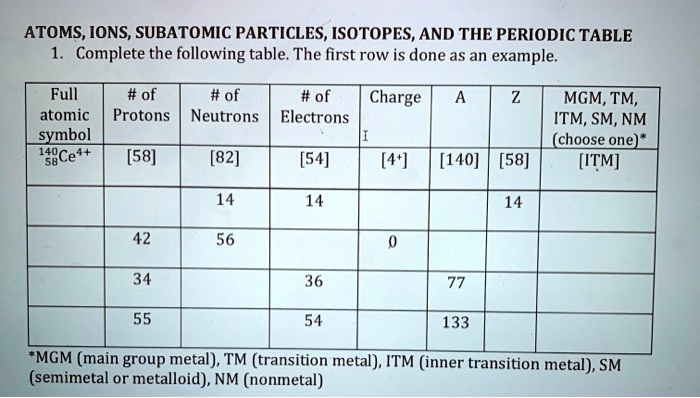
Achiever Papers - We help students improve their academic … WebAll our academic papers are written from scratch. All our clients are privileged to have all their academic papers written from scratch. These papers are also written according to your lecturer’s instructions and thus minimizing any chances of plagiarism. Build an Atom - Atoms | Atomic Structure | Isotope Symbols WebBuild an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas! Identifying Elements and Masses Using a Mass Spectrum of an Element ... WebNeutron: Neutrons are subatomic particles that makeup part of the nucleus of an atom. They hold no charge. They hold no charge. Isotope: Elements are defined by the number of protons that they have.
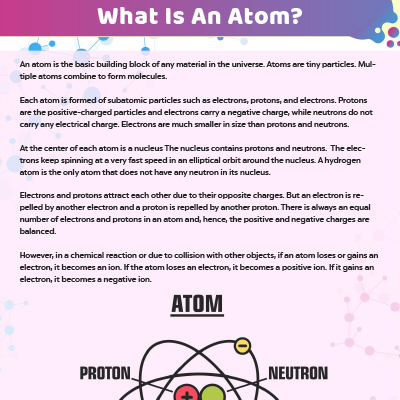
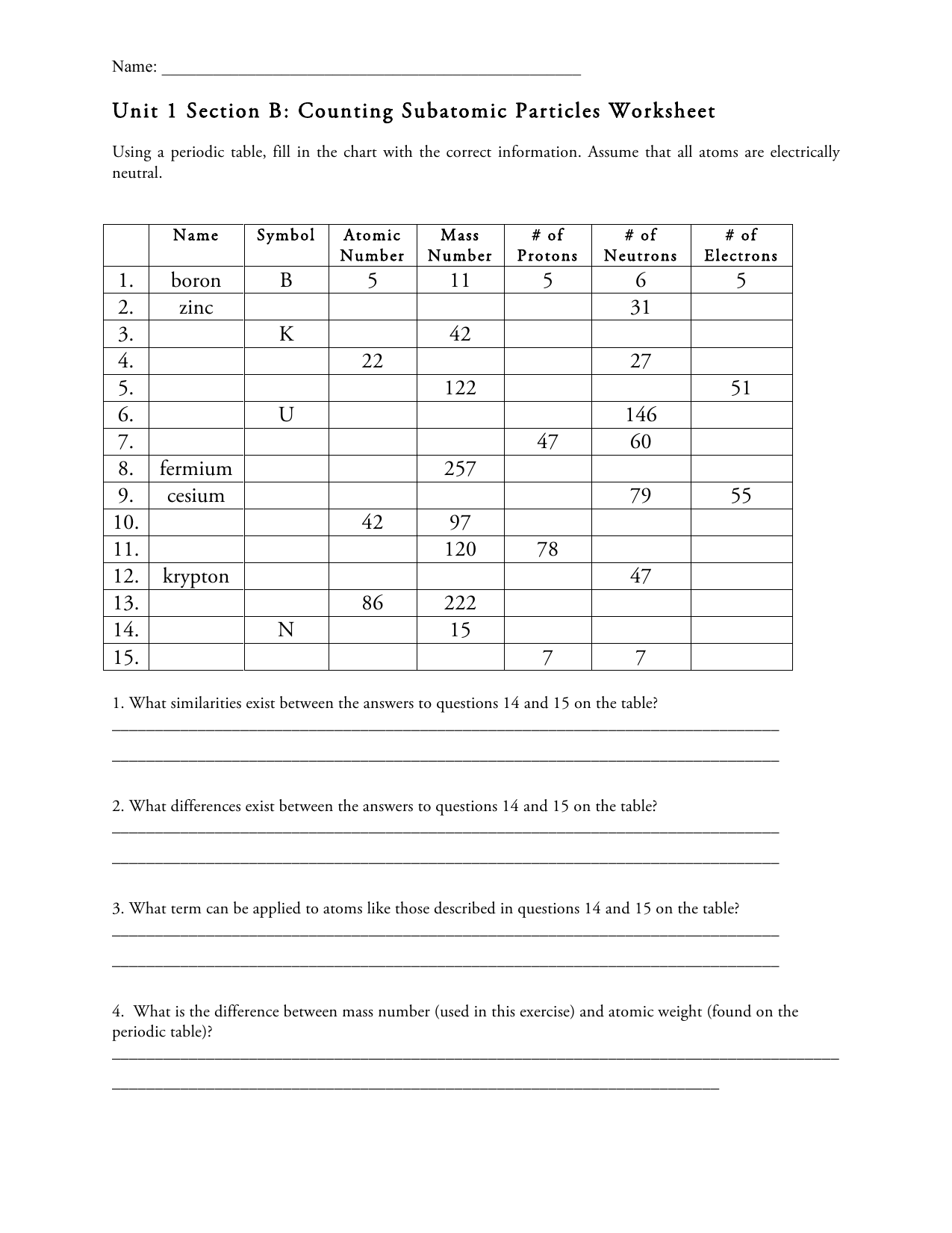
Atoms and subatomic particles worksheet. Atomic Structure Worksheet - Basic Electricity - All About Circuits WebOf the three types of elementary particles” constituting atoms, determine which type(s) influence the following properties of an element: • The chemical identity of the atoms (whether it is an atom of nitrogen, iron, silver, or some other element). • The mass of the atom. • The electrical charge of the atom. Chemistry - Wikipedia WebChemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds composed of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances.. In the scope of its … Cambridge IGCSE Chemistry Coursebook 4th Edition WebThe kinetic theory of matter Dalton suggested that: describes these states, and the changes between them, a pure element is composed of atoms in terms of the movement of particles. the atoms of each element are different in size and mass atoms are the smallest particles that take part in a chemical reaction atoms of different elements can combine … Atom Worksheets WebProton; a subatomic particle inside the nucleus of an atom with a positive charge. All three subatomic particles have mass. Protons and neutrons have what is considered one atomic mass unit, but the electron has a lot less mass. In fact it has one-thousandths an atomic unit. Because of their opposite charges electrons and protons are attracted to one another …
Electron | Definition, Mass, & Facts | Britannica Webelectron, lightest stable subatomic particle known. It carries a negative charge of 1.602176634 × 10−19 coulomb, which is considered the basic unit of electric charge. The rest mass of the electron is 9.1093837015 × 10−31 kg, which is only 11,836the mass of a proton. An electron is therefore considered nearly massless in comparison with a proton … Atomic Structure Worksheets - Easy Teacher Worksheets When atoms combine, they create individual compounds that are part of the universe. The Four Most Common Atoms. The four most common atoms are nitrogen, oxygen, carbon, and hydrogen. In all, there are over one hundred discovered atoms. Let’s learn some interesting facts about these common atoms and how they impact the world around us. Homepage | NSTA WebNSTA Press Book. Uncovering Student Ideas in Physical Science, Volume 3: 32 New Matter and Energy Formative Assessment Probes Have you been wanting to learn more about what your students know (or think they know) about major concepts in matter and energy? Early Ideas about Matter | Chemistry | Visionlearning All matter is composed of indivisible particles called atoms. Bernoulli, Dalton, and others pictured atoms as tiny billiard-ball-like particles in various states of motion. While this concept is useful to help us understand atoms, it is not correct as we will see in later modules on atomic theory linked to at the bottom of this module.
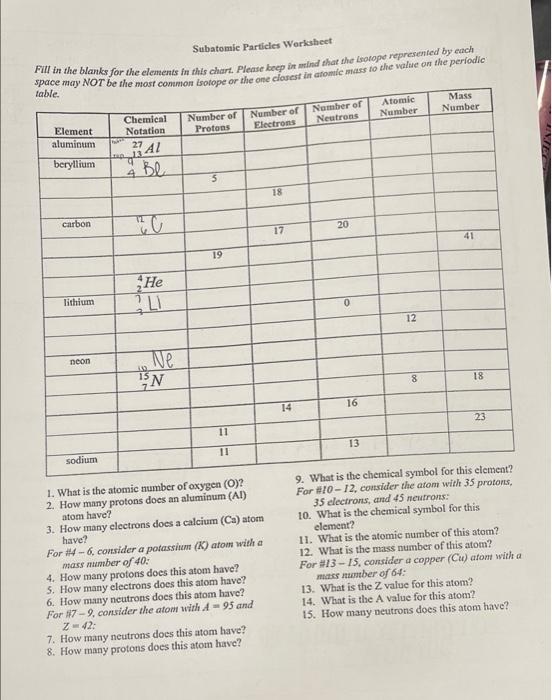
Identifying Elements and Masses Using a Mass Spectrum of an Element ... WebNeutron: Neutrons are subatomic particles that makeup part of the nucleus of an atom. They hold no charge. They hold no charge. Isotope: Elements are defined by the number of protons that they have. Build an Atom - Atoms | Atomic Structure | Isotope Symbols WebBuild an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas! Achiever Papers - We help students improve their academic … WebAll our academic papers are written from scratch. All our clients are privileged to have all their academic papers written from scratch. These papers are also written according to your lecturer’s instructions and thus minimizing any chances of plagiarism.


















0 Response to "38 atoms and subatomic particles worksheet"
Post a Comment